Gladstone NOW: The Campaign Join Us on the Journey✕

Scientists at Gladstone, along with collaborators, have solved the mystery of unusual blood clotting and inflammation in COVID-19—and identified a promising therapeutic strategy. Pictured from left: Gladstone scientists Zhaoqi Yan, Jae Ryu, Mauricio Montano, and Rahul Suryawanshi, who are are among the first authors of the new study in Nature.
In a study that reshapes what we know about COVID-19 and its most perplexing symptoms, scientists have discovered that the blood coagulation protein fibrin causes the unusual clotting and inflammation that have become hallmarks of the disease, while also suppressing the body’s ability to clear the virus. Importantly, the team also identified a new antibody therapy to combat all of these deleterious effects.
Published in Nature, the study by Gladstone Institutes and collaborators overturns the prevailing theory that blood clotting is merely a consequence of inflammation in COVID-19. Through experiments in the lab and with mice, the researchers show that blood clotting is instead a primary effect, driving other problems—including toxic inflammation, impaired viral clearance, and neurological symptoms prevalent in those with COVID-19 and long COVID.
The trigger is fibrin, a protein in the blood that normally enables healthy blood coagulation, but has previously been shown to have toxic inflammatory effects. In the new study, scientists found that fibrin becomes even more toxic in COVID-19 as it binds to both the virus and immune cells, creating unusual clots that lead to inflammation, fibrosis, and loss of neurons.
“Knowing that fibrin is the instigator of inflammation and neurological symptoms, we can build a new path forward for treating the disease at the root,” says Katerina Akassoglou, PhD, a senior investigator at Gladstone and the director of the Center for Neurovascular Brain Immunology at Gladstone and UC San Francisco. “In our experiments in mice, neutralizing blood toxicity with fibrin antibody therapy can protect the brain and body after COVID infection.”
From the earliest months of the pandemic, irregular blood clotting and stroke emerged as puzzling effects of COVID-19, even among patients who were otherwise asymptomatic. Later, as long COVID became a major public health issue, the stakes grew even higher to understand the cause of this disease’s other symptoms, including its neurological effects. More than 400 million people worldwide have had long COVID since the start of the pandemic, with an estimated economic cost of about $1 trillion each year.
Flipping the Conversation
Many scientists and medical professionals have hypothesized that inflammation from the immune system’s rapid reaction to the COVID-causing virus is what leads to blood clotting and stroke. But even at the dawn of the pandemic in 2020, that explanation didn’t sound right to Akassoglou and her scientific collaborators.
“We know of many other viruses that unleash a similar cytokine storm in response to infection, but without causing blood clotting activity like we see with COVID,” says Warner Greene, MD, PhD, senior investigator and director emeritus at Gladstone, who co-led the study with Akassoglou.
“We began to wonder if blood clots played a principal role in COVID—if this virus evolved in a way to hijack clotting for its own benefit,” Akassoglou adds.

Virologist Warner Greene (left) and neuroscientist Katerina Akassoglou (right) teamed up to investigate the root cause of unusual blood clotting and neurological issues among people with COVID-19 and long COVID. Their study overturned the prevailing theory that clotting is merely a consequence of inflammation.
Indeed, through multiple experiments in mice, the researchers found that the virus spike protein directly binds to fibrin, causing structurally abnormal blood clots with enhanced inflammatory activity. The team leveraged genetic tools to create a specific mutation that blocks only the inflammatory properties of fibrin without affecting the protein’s beneficial blood-clotting abilities.
When mice were genetically altered to carry the mutant fibrin or had no fibrin in their bloodstream, the scientists found that inflammation, oxidative stress, fibrosis, and clotting in the lungs didn’t occur or were much reduced after COVID-19 infection.
In addition to discovering that fibrin sets off inflammation, the team made another important discovery: fibrin also suppresses the body’s “natural killer,” or NK, cells, which normally work to clear the virus from the body. Remarkably, when the scientists depleted fibrin in the mice, NK cells were able to clear the virus.
These findings support that fibrin is necessary for the virus to harm the body.
Mechanism Not Triggered by Vaccines
The fibrin mechanism described in the paper is not related to the extremely rare thrombotic complication with low platelets that has been linked to adenoviral DNA COVID-19 vaccines, which are no longer available in the U.S.
By contrast, in a study of 99 million COVID-vaccinated individuals led by The Global COVID Vaccine Safety Project, vaccines that leverage mRNA technology to produce spike proteins in the body exhibited no excessive clotting or blood-based disorders that met the threshold for safety concerns. Instead, mRNA vaccines protect from clotting complications otherwise induced by infection.
Protecting the Brain
Akassoglou’s lab has long investigated how fibrin that leaks into the brain triggers neurologic diseases, such as Alzheimer’s disease and multiple sclerosis, essentially by hijacking the brain’s immune system and setting off a cascade of harmful, often irreversible, effects.
The team now showed that in COVID-infected mice, fibrin is responsible for the harmful activation of microglia, the brain’s immune cells involved in neurodegeneration. After infection, the scientists found fibrin together with toxic microglia and when they inhibited fibrin, the activation of these toxic cells in the brains of mice was significantly reduced.
“Fibrin that leaks into the brain may be the culprit for COVID-19 and long COVID patients with neurologic symptoms."
“Fibrin that leaks into the brain may be the culprit for COVID-19 and long COVID patients with neurologic symptoms, including brain fog and difficulty concentrating,” Akassoglou says. “Inhibiting fibrin protects neurons from harmful inflammation after COVID-19 infection.”
The team tested its approach on different strains of the virus that causes COVID-19, including those that can infect the brain and those that do not. Neutralizing fibrin was beneficial in both types of infection, pointing to the harmful role of fibrin in brain and body in COVID-19 and highlighting the broad implications of this study.
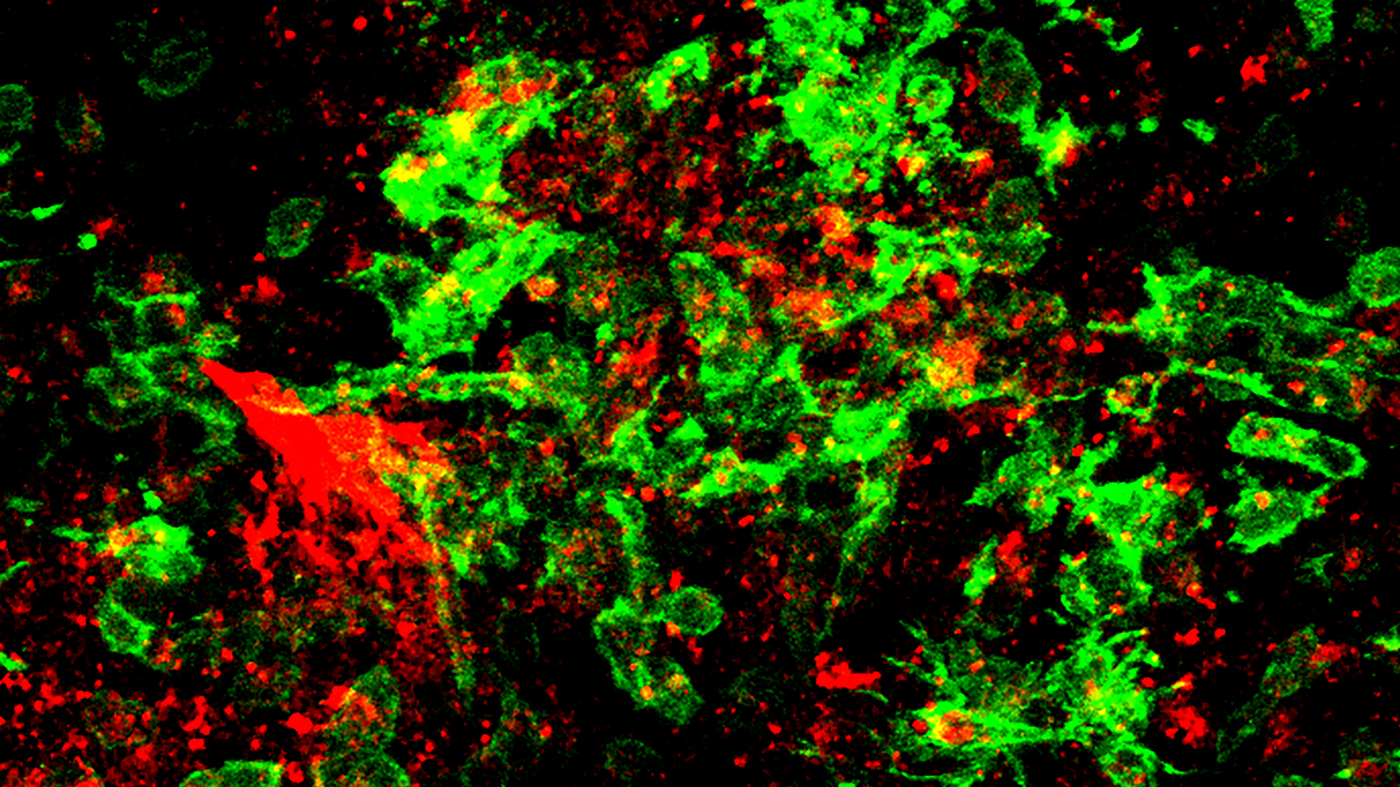
Fibrin (red) and toxic microglia (green) in the brain of a mouse infected with COVID-19. Image courtesy of Jae Kyu Ryu and Katerina Akassoglou.
A New Potential Therapy
This study demonstrates that fibrin is damaging in at least two ways: by activating a chronic form of inflammation and by suppressing a beneficial NK cell response capable of clearing virally infected cells.
“We realized if we could neutralize both of these negative effects, we could potentially resolve the severe symptoms we’re seeing in patients with COVID-19 and possibly long COVID,” Greene says.
Akassoglou’s lab previously developed a drug, a therapeutic monoclonal antibody, that acts only on fibrin’s inflammatory properties without adverse effects on blood coagulation and protects mice from multiple sclerosis and Alzheimer’s disease.
In the new study, the team showed that the antibody blocked the interaction of fibrin with immune cells and the virus. By administering the immunotherapy to infected mice, the team was able to prevent and treat severe inflammation, reduce fibrosis and viral proteins in the lungs, and improve survival rates. In the brain, the fibrin antibody therapy reduced harmful inflammation and increased survival of neurons in mice after infection.
“The fibrin immunotherapy can be tested as part of a multipronged approach, along with prevention and vaccination, to reduce adverse health outcomes from long COVID."
A humanized version of Akassoglou’s first-in-class fibrin-targeting immunotherapy is already in Phase 1 safety and tolerability clinical trials in healthy people by Therini Bio. The drug cannot be used on patients until it completes this Phase 1 safety evaluation, and then would need to be tested in more advanced trials for COVID-19 and long COVID.
Looking ahead to such trials, Akassoglou says patients could be selected based on levels of fibrin products in their blood—a measure believed to be a predictive biomarker of cognitive impairment in long COVID.
“The fibrin immunotherapy can be tested as part of a multipronged approach, along with prevention and vaccination, to reduce adverse health outcomes from long COVID,” Greene adds.
The Power of Team Science
The study’s findings intersect the scientific areas of immunology, hematology, virology, neuroscience, and drug discovery—and required many labs across institutions to work together to execute experiments required to solve the blood-clotting mystery. Akassoglou founded the Center for Neurovascular Brain Immunology at Gladstone and UCSF in 2021 specifically for the purpose of conducting multidisciplinary, collaborative studies that address complex problems.
“I don’t think any single lab could have accomplished this on their own,” says Melanie Ott, MD, PhD, director of the Gladstone Institute of Virology and co-author of the study, noting important contributions from teams at Stanford, UC San Francisco, UC San Diego, and UCLA. “This tour-de-force study highlights the importance of collaboration in tackling these big questions.”
Not only did this study address a big question, but it did so in a way that paves a clear clinical path for helping patients who have few options today, says Lennart Mucke, MD, director of the Gladstone Institute of Neurological Disease.
“Neurological symptoms of COVID-19 and long COVID can touch every part of a person’s life, affecting cognitive function, memory, and even emotional health,” Mucke says. “This study presents a novel strategy for treating these devastating effects and addressing the long-term disease burden of the SARS-CoV-2 virus.”
For Media
Kelly Quigley
Director, Science Communications and Media Relations
415.734.2690
Email
About the Study
The study, “Fibrin Drives Thromboinflammation and Neuropathology in COVID-19,” appears in the August 28, 2024 advanced online publication of Nature. Authors are Jae Kyu Ryu, Zhaoqi Yan, Mauricio Montano, Elif Sozmen, Karuna Dixit, Rahul K. Suryawanshi, Yusuke Matsui, Ekram Helmy, Prashant Kaushal, Sara Makanani, Thomas J. Deerinck, Anke Meyer-Franke, Pamela Rios Coronado, Troy Trevino, Min-Gyoung Shin, Reshmi Tognatta, Yixin Liu, Renaud Schuck, Lucas Le, Hisao Miyajima, Andrew Mendiola, Nikhita Arun, Brandon Guo, Taha Taha, Ayushi Agrawal, Eilidh MacDonald, Oliver Aries, Aaron Yan, Olivia Weaver, Mark Petersen, Rosa Meza Acevedo, Maria del Pilar Alzamora, Reuben Thomas, Michela Traglia, Valentina Kouznetsova, Igor Tsigelny, Alexander Pico, Kristy Red-Horse, Mark Ellisman, Nevan Krogan, Mehdi Bouhaddou, Melanie Ott, Warner Greene, and Katerina Akassoglou.
This research was supported by Brightfocus Postdoc Fellowship Award, Kaganov Scholarship for Excellence in Neuroscience, National Institutes of Health, National Multiple Sclerosis Society, Howard Hughes Medical Institute, National Science Foundation, National Institute of Allergy and Infectious Diseases’ Host Pathogen Map Initiative, James B. Pendleton Charitable Trust, The Roddenberry Foundation, The Foundation for a Better World, Edward and Pearl Fein, Robert Hamwee, and Simon Family Trust.
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Featured Experts
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Gladstone scientists identified a cellular boundary that guides heart development and revealed how disrupting it can lead to holes in the heart’s wall.
News Release Research (Publication) Congenital Heart Disease Cardiovascular Disease Bruneau LabGene Editing Strategy Could Treat Hundreds of Inherited Diseases More Effectively
Gene Editing Strategy Could Treat Hundreds of Inherited Diseases More Effectively
Scientists at Gladstone show the new method could treat the majority of patients with Charcot-Marie-Tooth disease.
News Release Research (Publication) Neurological Disease Conklin Lab CRISPR/Gene EditingGenomic Maps Untangle the Complex Roots of Disease
Genomic Maps Untangle the Complex Roots of Disease
Findings of the new study in Nature could streamline scientific discovery and accelerate drug development.
News Release Research (Publication) Marson Lab Genomics Genomic Immunology