Gladstone NOW: The Campaign Join Us on the Journey✕
Gene editing and stem cells are working in tandem at Gladstone to change science and medicine.
Here’s the future: a doctor takes a blood sample from her sick patient. In the lab down the hallway, scientists reset the patient’s blood cells into a developmental blank slate using a cocktail of chemicals. Then, they add a combination of tiny molecules that edit the cells’ DNA. Perhaps this editing process repairs a disease-causing mutation or gives the cells a new ability to ward off infection, inflammation, or cancer. Finally, the embryo-like cells are grown into healthy, adult cells—heart cells to quell heart disease, brain cells to treat Alzheimer’s, or immune cells to shrink a tumor—and infused back into the patient’s body. Or perhaps, the doctor infuses the specially designed molecules that edit cells’ DNA right into the patient, and corrections are made directly inside their body.
Twenty years ago, this kind of living medicine would have sounded like the makings of a science fiction story.

In his lab, Deepak Srivastava uses iPS cell technology to study diseased heart cells and determine how certain genetic mutations lead to developmental heart defects.
Now, not only can we read the genomic codes of cells, we can rewrite the code too.
“Now, not only can we read the genomic codes of cells, we can rewrite the code too—and do it in human cells,” says Deepak Srivastava, MD, president of Gladstone Institutes. “At Gladstone, we’re embedding both iPS cells and CRISPR into world-class research. We have scientists actively and creatively thinking about how these technologies can be used, and how they intersect with each other, to come up with new ways to prevent, treat, and even cure diseases.”
A Path to Patient Cells
In 2006, Gladstone Senior Investigator Shinya Yamanaka, MD, PhD,discovered that mature cells from an adult—such as those collected in a routine blood sample—can be reprogrammed into stem cells using a precise mixture of proteins (known as the Yamanaka factors). These reprogrammed stem cells are called induced pluripotent stem cells, or iPS cells. And, like cells in an early embryo, they are capable of developing into nearly any cell in the body, a condition called pluripotency.
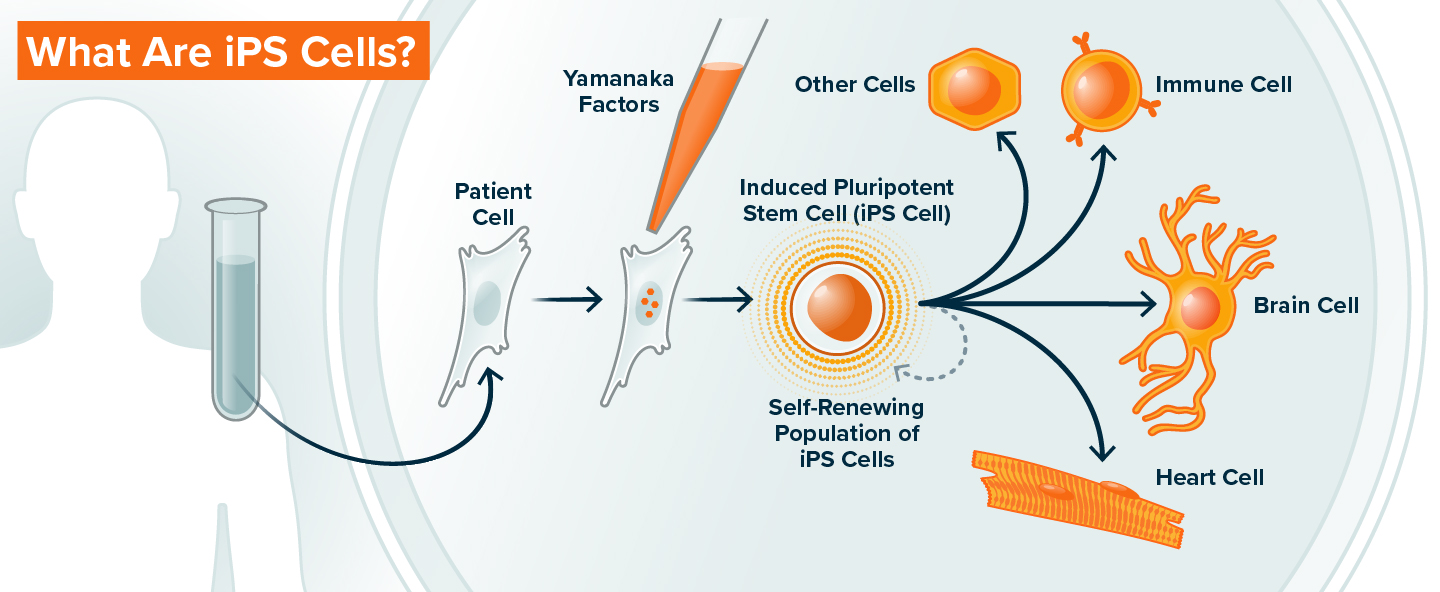
Cells of the early embryo have pluripotency, which is the capacity to turn into almost any other kind of cell in the human body. Induced pluripotent stem (iPS) cells are cells derived from adults that are reset to have this same potential. Patients donate blood or skin cells, and upon the introduction of small number of molecules—the “Yamanaka factors”—the cells lose their adult characteristics and begin to resemble immature, embryonic cells. In this state, iPS cells can make copies of themselves indefinitely, or be programmed to become heart cells, neurons, immune cells, or any other cell type in the body. These patient-derived cells can be used to study how human organs develop, and because iPS cells have exactly the same genome as the patient from whom they are derived, they can also be used to study genetic diseases and develop new therapies.
Until Yamanaka’s breakthrough, scientists who wanted to study adult human cells relied on cells directly from the body. This means they needed to collect the specific type of cell they wanted to study, which is excessively difficult for many cell types, like brain and heart cells. Alternatively, they could obtain cells from human embryos, although these are limited in supply and present ethical challenges.
Bruce Conklin, MD, senior investigator at Gladstone, recalls using embryonic stem cells around the turn of the millennium to study inherited heart disease. He wanted to pinpoint differences in how heart cells of healthy and diseased people functioned. But since the stem cells were from human embryos—not adults who could show symptoms of heart disease—his team struggled.
With iPS cells, we suddenly had the opportunity to take cells from a patient that we could fully examine.
“We never knew whether any of these embryonic stem cells would have gone on to form a healthy adult human,” says Conklin. “With iPS cells, we suddenly had the opportunity to take cells from a patient that we could fully examine. We knew with certainty that any cells we generated were from a healthy or diseased individual.”

Shinya Yamanaka discovered how to reprogram adult cells into induced pluripotent stem cells (iPS cells), which are capable of developing into nearly any cell in the body.
Yamanaka presented his initial data on iPS cells in 2006, and Conklin immediately saw the promise. Eager to move his research into iPS cells, Conklin rapidly submitted regulatory paperwork. His study, which coaxed blood cells from patients into beating heart cells, was the first approved laboratory study in San Francisco to use human iPS cells.
Soon after Yamanaka published his findings in the journal Cell, Srivastava also began to carry out similar experiments, trying to determine how certain genetic mutations lead to developmental heart defects. He had already discovered several genes that were implicated in some heart defects, but not how variants of those genes changed the function of heart cells—there had been no way to obtain diseased cells from patients’ hearts.
“It was the advent of iPS cell technology that allowed us, for the first time, to study diseased human heart cells,” says Srivastava. “We could now use blood samples from patients we had studied genetically to make lots of different kinds of heart cells in the lab—it opened up a whole new avenue to figure out which cells were causing trouble for a patient and why.”
Precise Edits Change the Game
After Yamanaka’s discovery, iPS cells became invaluable for basic researchers like Conklin and Srivastava. But it remained tricky to identify which parts of the genome were responsible for a patient’s disease.
“We could compare iPS cells from a patient and a healthy volunteer, but there would often be far too many genetic differences between the two individuals to pin down which gene was causing disease,” explains Yamanaka.
Jennifer Doudna, PhD, a professor at UC Berkeley and now also a Gladstone senior investigator, would help change that by discovering a technology to edit the genome
Her lab studied how bacteria protect themselves from invading viruses by using a system known as CRISPR to cut up a virus’s DNA at specific sites. In 2012, with collaborator Emmanuelle Charpentier, Doudna showed how CRISPR could be harnessed to make changes to the DNA in any cell.
This is the perfect example of what can happen when scientists go after questions that are of interest from a fundamental perspective.
“This is the perfect example of what can happen when scientists go after questions that are of interest from a fundamental perspective, and the science goes in unexpected directions,” says Doudna.
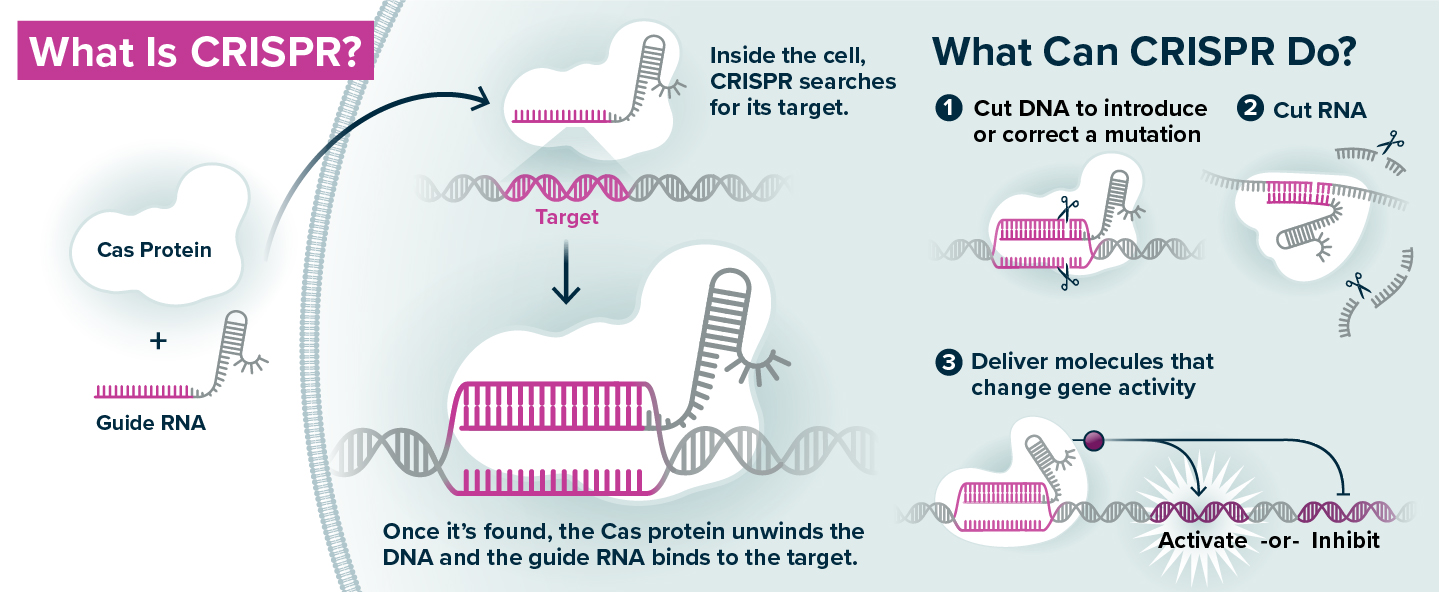
CRISPR genome editing is a technology that repurposes components of an ancient, bacterial immune system. The key elements are the Cas protein, which can cut DNA, and the guide RNA, which has a sequence identical to a specific stretch of the genome. When a guide RNA and Cas protein are introduced to a cell, the guide RNA directs the Cas protein to the area of the genome it matches (like a zip code), so that the DNA can be cut in that specific location to introduce a mutation. There are also versions of the CRISPR system that use the guide RNA to direct delivery of modified Cas proteins that turn on or off a gene, rather than cutting the DNA sequence, or alternative Cas proteins that cut RNA rather than DNA.
The CRISPR technology is composed of two main elements. The first is a protein called Cas9, which acts like a pair of scissors that can cut DNA. The second is an RNA molecule (known as guide RNA) that matches a stretch of the DNA in the cell’s genome.
Scientists can design the guide RNA to recognize a specific DNA sequence; the guide RNA brings the Cas protein to that sequence (like a zip code), and then the Cas protein cuts the DNA in that exact location. Once the cut is made, the cell repairs the DNA either by mending together the broken ends, or by pasting in a new piece of DNA at the site of the cut. It is a programmable system that can re-write the genetic code with great specificity.
Once it became clear that CRISPR is a tool that can be used to manipulate DNA sequences in any cell type, it was a total paradigm shift.
 Jennifer Doudna showed how CRISPR could be harnessed to make changes to the DNA in any cell—a technology that has allowed scientists to manipulate a cell's genome in entirely new ways.
Jennifer Doudna showed how CRISPR could be harnessed to make changes to the DNA in any cell—a technology that has allowed scientists to manipulate a cell's genome in entirely new ways.
“Once it became clear that CRISPR is a tool that can be used to manipulate DNA sequences in any cell type, it was a total paradigm shift,” says Doudna. “That makes it a very impactful technology that’s now being utilized globally for everything from human health diagnostics to addressing some of the challenges of climate change.”
Where iPS cells had provided a way to generate any cell type, CRISPR provided a way to manipulate those cells in new ways.
“CRISPR was really a game-changer for iPS cells,” says Yamanaka. “With CRISPR, we can edit a mutation in a patient and see if it makes a difference in a cell’s function.”
One way to determine what a gene does is to break it and observe the consequences on the health of a cell or organism. Scientists had worked out a few methods to disrupt gene sequences in the past, but those approaches were inefficient and imprecise. The discovery of CRISPR meant that researchers suddenly had a fast and accurate way to disrupt the genes they were studying and then study the effects.
“When CRISPR came along, it immediately became clear that this was 10- to 100-fold better than any gene editing technology that had come before,” says Conklin.
The Power of CRISPR
Over the past decade, CRISPR has evolved into more than a tool to cut DNA. Researchers have made variations to the technology that can also turn on or off every gene in the genome, letting them study the impact of each gene on a particular cellular behavior.
For example, Alex Marson, MD, PhD, director of the Gladstone-UCSF Institute of Genomic Immunology, has worked with Doudna to apply CRISPR to immune cells (which includes T cells) in order to improve cell-based therapies.
"It’s exciting to see that CRISPR has opened the door to working on new types of organisms and tissues,” says Doudna. “T cells are a great example, because in the past, it was nearly impossible to manipulate them genetically.”
Marson uses the technology to alter thousands of genes in human immune cells and determine which genes are responsible for which of the cells’ functions.
“We take a human blood sample, which includes millions of T cells, and we use CRISPR to introduce different genomic changes to each cell,” Marson explains. “Then, we compare the cells against each other and see which have the properties we want.”
Marson’s research has uncovered new ways to genetically program immune cells to be more adept at finding and killing cancer cells using CRISPR.

Alex Marson (right)—seen here with scientist Zachary Steinhart (left)—uses CRISPR technology to improve cell-based immunotherapies.
“We’re at a point now where we’re really installing whole new genetic programs into cells with CRISPR,” says Marson.
Eventually, Marson imagines these kinds of gene-edited immune cells will be useful for treating a plethora of other conditions
“We’re trying to think broadly about what types of instructions we give to different types of cells and how that can make the cells more effective for treating more and more diseases, such as autoimmune, inflammatory, and infectious diseases,” he says. “Maybe in the future we’ll even program immune cells to travel to specific sites in the body to treat neurodegeneration or cardiovascular disease.”
Already, Gladstone Senior Investigator Yadong Huang, MD, PhD, and his team are using CRISPR to edit genes related to Alzheimer’s disease risk. The genetic variant known as ApoE4, for instance, is the strongest known risk factor gene for the disease.

Yadong Huang and his team are using CRISPR to edit genes related to Alzheimer’s disease risk.
Huang has shown that, in neurons derived from iPS cells, editing or removing ApoE4 with CRISPR prevents the neurons from developing molecular signs of Alzheimer’s disease. His group is now planning studies in animals to test the efficacy of using CRISPR to edit ApoE4 and reduce Alzheimer’s risk.
“CRISPR has made it possible for us to do gene editing work in human brain cells derived from iPS cells in order to study Alzheimer’s and develop potential gene therapies for treating ApoE4-related Alzheimer’s disease,” says Huang.
CRISPR has diverse biological applications far beyond these types of genetic screens or cell therapies, though. In 2020, a team of scientists including Doudna and Melanie Ott, MD, PhD, director of the Gladstone Institute of Virology, used CRISPR to create a diagnostic test for the SARS-CoV-2 virus.
The scientists first turned to a unique Cas enzyme, which cuts RNA instead of DNA, because SARS-CoV-2 has an RNA genome. They then combined this alternative Cas enzyme with a molecule that becomes fluorescent when cut, along with a guide RNA that recognizes the SARS-CoV-2 genome. When this cocktail is mixed with a patient sample from a nasal swab, the guide RNA detects whether RNA from the SARS-CoV-2 virus is present. If so, it activates Cas, which in turn cuts the fluorescent molecule and causes it to light up, indicating that the swab tested positive for the virus.
The test is extremely sensitive and detects low levels of the virus, like PCR tests, but is very quick and simple, like the rapid at-home antigen tests used for COVID-19.
“This test can be very rapidly adapted to test for any RNA virus in the future,” says Ott. “Before the COVID-19 pandemic, we were originally developing this test for HIV, and we’re now doing work to create tests that can simultaneously detect multiple different viruses, like influenza and RSV.”
A Convergence for Understanding Disease
While those applications of CRISPR don’t necessarily intersect with stem cells, the convergence of the technology with iPS cells is particularly powerful for understanding disease.

By combining CRISPR and iPS cell technologies with the robotic microscopes he developed, Steve Finkbeiner hopes to unravel the underlying mechanisms behind diseases like Alzheimer's, Parkinson's, and ALS.
Steve Finkbeiner, MD, PhD, director of the Center for Systems and Therapeutics at Gladstone, is merging new technology with patient-derived iPS cells and CRISPR to learn about neurological diseases like Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS).
For most people with these diseases, there is no known genetic cause. That makes it impossible to create animal models of the diseases that fully reflect human cases. But Finkbeiner’s team can isolate cells from patients with the diseases, convert them into neurons, and study them. And in cases where the disease is caused by a genetic mutation, they use CRISPR to correct the mutation and create control lines of iPS cells. By comparing neurons derived from patients with control iPS cells, they can determine precisely how the mutation derails the neurons’ biology
Finkbeiner has also developed robotic microscopes that can track changes in cells over time, as well as artificial intelligence platforms to analyze this microscope data
By combining all these technologies, we’re hopeful for the first time that we can begin to unravel the underlying mechanisms behind diseases.
“By combining all these technologies, we’re hopeful for the first time that we can begin to unravel the underlying mechanisms behind these diseases,” he says. “Having a large community at Gladsome focused on iPS cells and CRISPR is really helpful because a very large knowledge base has been developed and the expertise is broadly shared, which makes it easier and more successful for us to use these approaches and adopt innovations at an early stage.”
Doudna, for her part, recently launched a study that uses iPS cells to probe the genetics of a rare, inherited disease that predisposes people to many tumors. Her research group collects cells from patients and from their relatives who are unaffected by the disease. Then, the scientists transform the cells into a variety of adult cell types affected by the disease and use CRISPR to edit genes that might be related. In each cell type, they can study whether the edited genes prevent the formation of tumors, helping them pinpoint the genes responsible for disease, which could potentially be targeted by a new therapy
That workflow is becoming increasingly common.
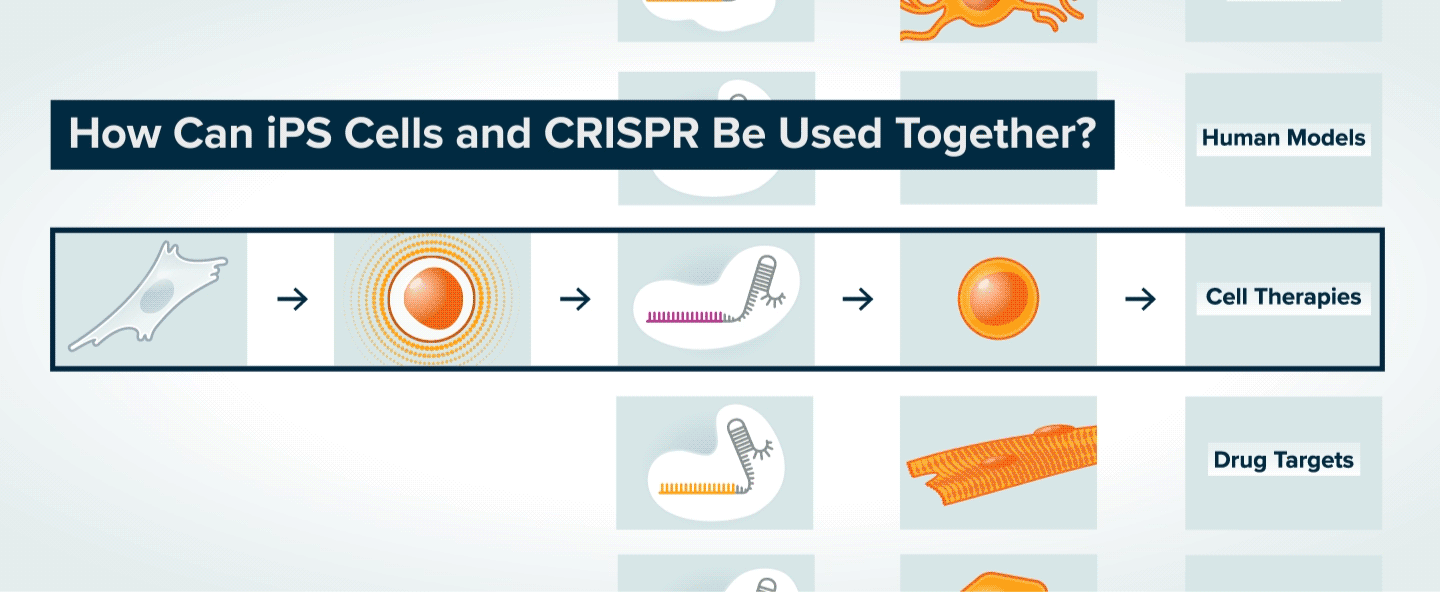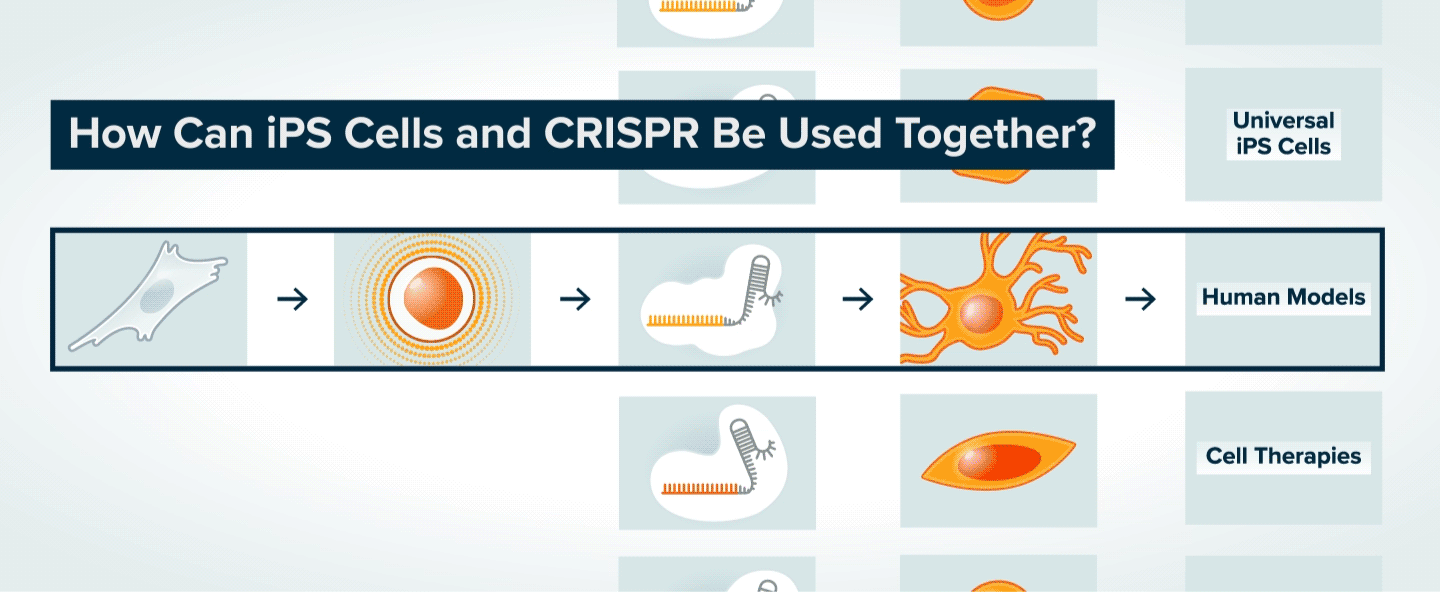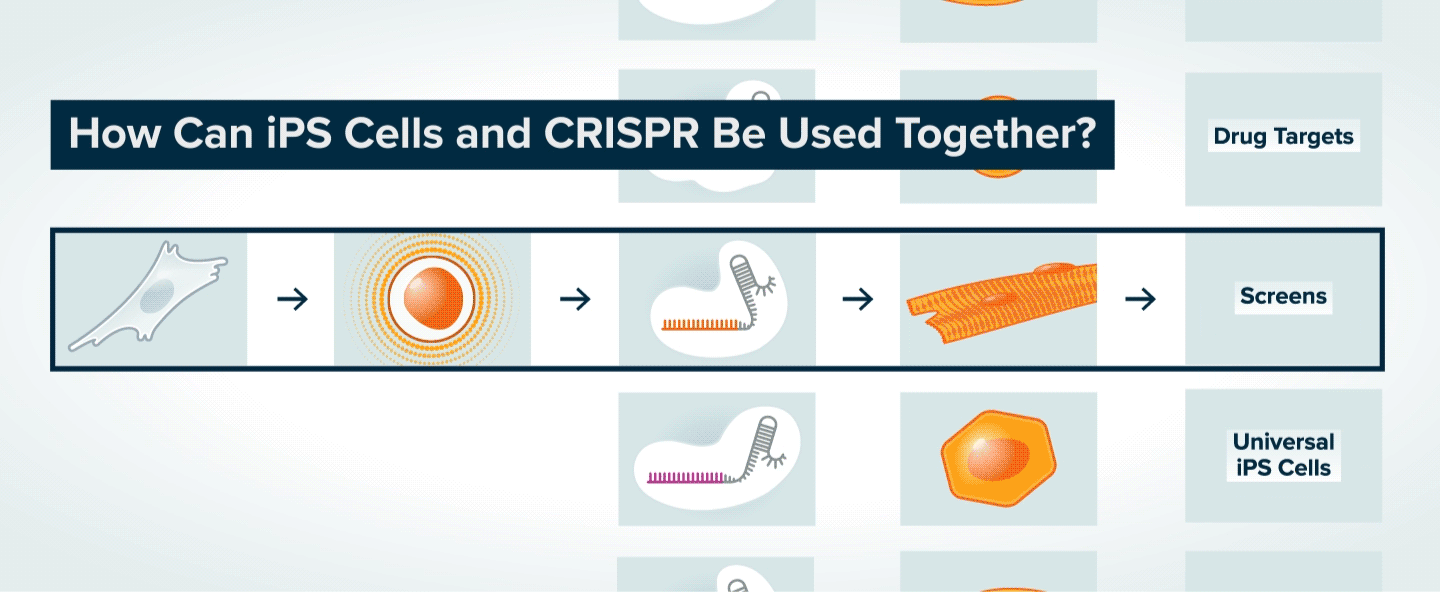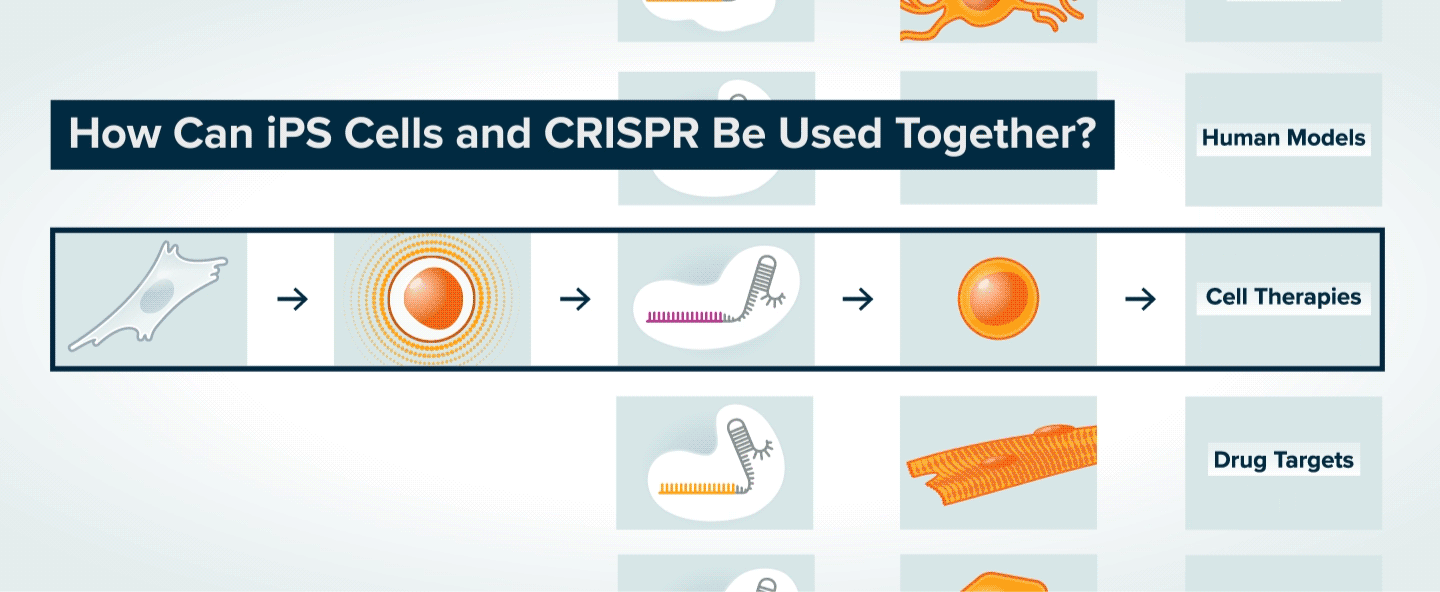
Once patient skin or blood cells have been reprogrammed into iPS cells, CRISPR can be used to make specific genetic changes in those cells, such as introducing or correcting a mutation, or turning on or off a gene. The iPS cells can then be transformed into various types of adult cells. The modified adult cells are useful to researchers interested in understanding the role of specific genes in disease and the potential to treat disease by making specific genetic changes. The cells can also be used to generate tissue for cell therapy, or to search for new therapeutics.
In 2020, Srivastava’s team used a similar tack to find a potential treatment for calcific aortic valve disease, the third leading cause of heart disease. His team had previously identified a key genetic cause of the disease by studying a large family. The researchers then converted patient cells into iPS cells, and then into the cells that line the valve of the heart. Next, they used CRISPR to edit genes in the cells and determine which could prevent the calcification seen in people with disease.
Their analysis of the data using artificial intelligence pointed to a genetic network that could be targeted with a pharmaceutical compound to treat the disease. Indeed, this drug prevented and arrested disease in mice. Srivastava is now moving the compound toward early clinical trials.
“This was an example of what happens when we can combine patient-derived iPS cells, CRISPR technology, and powerful data science,” he says.
“For COVID-19, this was immensely helpful because of the wide variety of tissue types that the SARS-CoV-2 virus can infect,” says Ott. “This kind of study using iPS cells can really help us get a handle on what’s going on during infection at a cellular level.”
CRISPR to Cure Disease
Although some studies rely on the power of CRISPR only as a research tool, therapies using this technology may also soon be available to patients. The U.S. Food and Drug Administration has approved, for instance, multiple clinical trials that use CRISPR to replace the defective gene that causes sickle cell disease, an inherited blood disorder affecting millions of people around the world. The treatment typically involves removing cells from patients, editing the genes inside the cells in the lab, and then infusing them back into the patient.
Innovative Genomics Institute, is helping lead one of these Phase I trials, which is currently enrolling patients at three different University of California campuses.
“We’re the only non-profit organization running a trial like this,” she says. “It’s important to us that as CRISPR therapies advance to the clinic, we’re helping ensure the technology is accessible, affordable, and responsibly used.”

Jennifer Doudna is helping advance CRISPR therapies to the clinic in order to ensure the technology is accessible, affordable, and responsibly used.
With that in mind, her group’s trial doesn’t require the removal of bone marrow from patients with sickle cell disease. Instead, it puts the molecules needed for CRISPR directly into the bloodstream—this difference sets it apart from other commercial ventures.
By delivering CRISPR directly to patients, without a transplant, we immediately remove weeks of hospitalization and a lot of the costs.
“By delivering CRISPR directly to patients, without a transplant, we immediately remove weeks of hospitalization and a lot of the costs, which is important given the current price tag of roughly $2 million per patient for gene therapies,” says Doudna.
For the most part, these kinds of CRISPR-based therapies are still years away. But at Gladstone and elsewhere, researchers are driven forward by their promise
Srivastava and his team, for example, now know what gene corrections can improve the signs of an inherited form of heart disease—if he can get the CRISPR system into the heart cells of living patients to make these edits, he thinks he can successfully treat them.
“At the moment, one of the biggest challenges with CRISPR therapies is delivering them to the exact cell types we’re targeting within the human body,” he says. “When you put CRISPR into the blood, most of it ends up in the liver, because that organ filters our blood. That’s not so great if your therapy is meant to target the heart. But many scientists and companies are working on new ways to overcome this.”
Similarly, Conklin is using the technology to try to treat motor neuron diseases, including Charcot-Marie-Tooth disease and amyotrophic lateral sclerosis (ALS). His ultimate goal is to deliver CRISPR into the spinal cord of patients to edit the genes that cause their disease. But first, he has to fine-tune some of the basics on how a CRISPR therapy could work, asking questions like exactly where to cut the DNA around a gene to remove or replace it.
“We need to design a CRISPR therapy that would work in most patients, even though each person has a slightly different DNA sequence,” explains Conklin. “In addition, we need to make sure DNA only gets cut in the precise locations we want, without any unintended edits to the genome.”
Even for HIV—a notoriously hard-to-treat virus that has stymied researchers for decades—CRISPR offers new hope.
 Melanie Ott (left) is using both iPS cells and CRISPR to study how viruses infect human cells.
Melanie Ott (left) is using both iPS cells and CRISPR to study how viruses infect human cells.
Even for HIV—a notoriously hard-to-treat virus that has stymied researchers for decades—CRISPR offers new hope. Ott and her colleagues are using CRISPR to permanently silence any copies of the virus left in people’s bodies after antiretroviral therapy. Rather than cut out the viral sequences, their approach combines the CRISPR system, which can find a particular gene sequence, with a signal that specifically turns genes off—a nuance that Ott thinks might appeal to people with HIV.
“When you talk to people who live with HIV, the idea of removing parts of their DNA can be scary to them,” says Ott. “But this idea of a drug that turns off a gene without making any cuts is more acceptable.”
A Path Toward Stem Cell Treatments
As CRISPR has sped toward the clinic in the decade since Doudna’s discovery, the use of iPS cells to treat disease is also getting closer to the clinical stages. Researchers believe that iPS cells transformed into adult cells in the lab can be used as a source of new cells or tissues. Clinical trials are ongoing for diseases such as Parkinson’s diseases, retinal diseases, corneal diseases, heart failure, and cancers, as well as for spinal cord injuries and knee cartilage defects.
In parallel, researchers are trying to overcome remaining challenges before they can bring iPS cell therapies to more patients at a commercial scale.

Bruce Conklin (right) is using CRISPR to design a therapy for motor neuron diseases that could be delivered into the spinal cord of patients to edit the genes that cause their disease.
“In some cases, we’re still struggling to make mature, pharmaceutical-grade final products with iPS cells,” says Yamanaka. “We do not transplant stem cells themselves; we first need to reprogram them into a specific cell type. And while we can make immature heart cells that behave like the ones in an embryo, for instance, it’s hard to make completely mature heart cells that are exactly like the ones in adult bodies.”
As scientists look for ways to generate exactly the types of cells they want from iPS cells, CRISPR could be a boon for tackling this challenge too.
In its native bacterial environment, the CRISPR system keeps an ordered log of the viral DNA it encounters as it attacks invading viruses—a kind of receipt book. Seth Shipman, PhD, a Gladstone associate investigator, has discovered how to use this kind of logbook to track the series of genetic events that occur during normal cellular development. With a readout of what happens naturally, Shipman thinks he can learn how to better manipulate iPS cells to become more like adult cells.
“What we ultimately want to do is play back this series of events in iPS cells, so that we can reprogram them with more precision into very specific cell types that behave the way they should,” says Shipman.

Seth Shipman (left) and his team, including Santiago Lopez (right), uses the CRISPR system to track the series of genetic events that occur during normal cellular development.
Even if Shipman can create new recipes for transforming iPS cells into the desired cell types, the field faces cost and accessibility issues. Ideal stem cell treatments would be custom-made for a patient to prevent their immune system from rejecting the new cells. That’s because each person has a unique mixture of tags on their cells that identify them as their own, and their body will reject cells with foreign tags.
Using CRISPR, Yamanaka is now trying to change that; he’s editing those immune tags in iPS cells in the hopes of creating “universal” iPS cells that wouldn’t be rejected by donors.
We want to genetically modify these cells so that potentially just a handful of lines of iPS cells could be used to generate cells that would work for the entire world population.
“Ultimately, we want to genetically modify these cells so that potentially just a handful of lines of iPS cells could be used to generate cells that would work for the entire world population,” he says.
This would be like creating a bank of iPS cells similar to a blood bank where people’s blood type are matched for blood transfusions.
“And we are already making clinical-grade genome-edited iPS cell lines—so it’s not fiction anymore,” adds Yamanaka. “The next steps will be to test their safety in clinical trials.”
If that research pans out, it could help iPS cells and CRISPR converge in the clinic. Marson, for instance, imagines that one day his CRISPR-edited immune cells could be an affordable, off-the-shelf product rather than a custom-made one for each person.
“We can potentially take a self-renewing population of iPS cells, introduce many CRISPR modifications, and grow them in large quantities for many patients,” Marson says.

Research by Seth Shipman (left)—seen here in the lab with Santiago Lopez (right)—could eventually help scientists learn how to better manipulate iPS cells.
Intersection at Gladstone
At Gladstone, both Doudna and Yamanaka have launched deep, long-term collaborations with researchers across fields to help their technologies grow, adapt, and treat human diseases. Their presence as founders of their respective technologies is one piece of why Gladstone is leading the convergence of iPS cells and CRISPR.
“Gladstone is really a nexus for where these types of transformational technologies can be fostered, and for where the technologies can in turn lead to discoveries and new treatments for disease,” says Marson
Alone, iPS cells and CRISPR have much to offer. But together, they are poised to change the face of biomedical research and human disease.
“We’ve reached a point where we can take a patient’s cell, turn it into whatever cell type we want, and then manipulate the genome of those cells in all these creative ways to treat diseases,” says Srivastava. “That’s truly revolutionary.”
Featured Experts
How Do Vaccines Work?
How Do Vaccines Work?
An in-depth explainer about different types of vaccines and what happens in the body after vaccination.
Gladstone Experts COVID-19 Deep DiveSolving the Aftermath of Brain and Spinal Cord Injuries
Solving the Aftermath of Brain and Spinal Cord Injuries
Gladstone scientists make headway toward new treatments for people with chronic long-term effects
Gladstone Experts Traumatic Brain Injury Neurological Disease Akassoglou Lab Paz Lab Srivastava Lab Deep DiveRe-Engineering the Human Immune System
Re-Engineering the Human Immune System
Researchers at the Gladstone-UCSF Institute of Genomic Immunology are learning how to harness the power of the human immune system in new ways
Gladstone Experts Cancer Genomic Immunology Carnevale Lab Doudna Lab Marson Lab Pelka Lab Roybal Lab Tang Lab Ye Lab