Gladstone NOW: The Campaign Join Us on the Journey✕
Researchers at the Gladstone-UCSF Institute of Genomic Immunology are learning how to harness the power of the human immune system in new ways.
Human immune cells are powerful agents of change—they can wipe out a virus, attack a cancer, or cause massive inflammation. But for most of history, the immune system has been largely uncontrollable. And, it can fail at its job—viruses can kill, cancers can evade immune attacks, and inflammation can cause chronic disease.
Two years ago, a team of multidisciplinary researchers from Gladstone Institutes and UC San Francisco (UCSF) came together with a lofty goal: learn how to precisely control human immune cells and turn them into living therapeutics. That’s when they launched the joint Gladstone-UCSF Institute of Genomic Immunology.
Director Alex Marson, MD, PhD, sees the institute as operating at the crux of two rapidly moving fields—immunology and genomics—and pulling threads from both disciplines into an integrated effort that can change the way diseases are treated. Already, clinicians use CAR-T cells, taken from patients’ bodies and genetically altered, to treat certain types of blood cancers. But Marson sees an even broader potential for therapies like this.
“If we understand what governs how immune cells function, we can start re-engineering them to turn them into living drug-delivery devices,” he says. “We can start imagining immune cells that are engineered to treat not just cancer, but neurodegenerative and cardiovascular diseases too.”
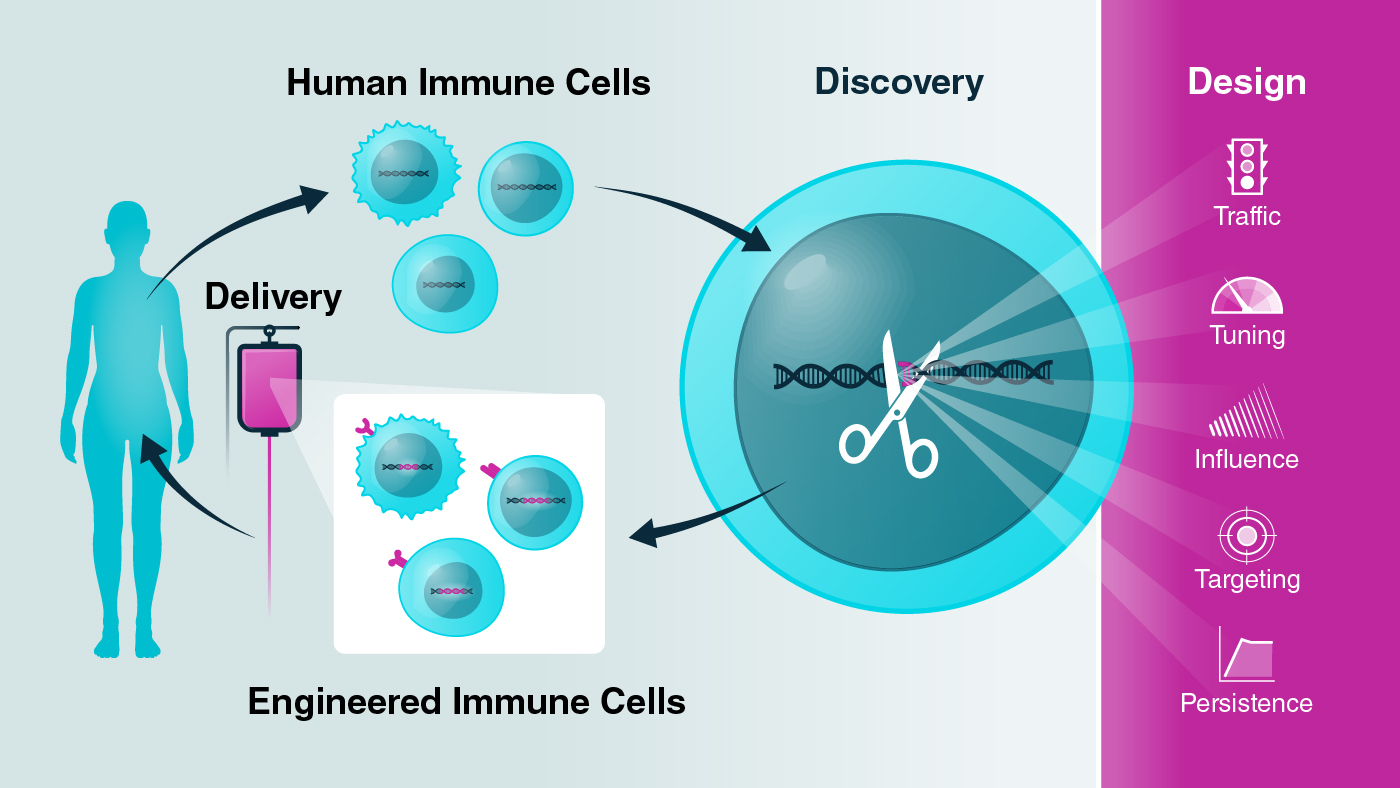
The Gladstone-UCSF Institute of Genomic Immunology was founded to deepen our understanding of the genes that control the immune system and to accelerate development of new immunotherapies for a wide range of diseases.
Immune cells are collected and analyzed from healthy individuals and patients with diseases ranging from cancer, to autoimmune diseases, to infections. Researchers harness the power of CRISPR to alter DNA across the genome and discover specific areas that control critical immune cell functions. These CRISPR studies are revealing a DNA vocabulary and grammar that will enable the team to write new DNA codes and design immune cells with improved functions. The scientists' goal is to deliver these engineered immune cells into patients to serve as "cellular medicines."
That kind of application is not yet ready for prime time, but over the past few years, Marson and his colleagues have made tremendous progress in developing new methods to alter the genes inside immune cells and uncover the networks of molecules that control immune cell activity. Those breakthroughs, they say, set the groundwork for re-engineering the human immune system.
“We now have this unprecedented suite of technologies that allow us to peer inside human cells and understand how the DNA sequence in each cell acts as a source code controlling its function,” says Marson. “And we can use some of these same technologies to start manipulating gene sequences to enable a range of therapeutic applications.”
A New Editing Toolbox
Over the last decade, CRISPR genome editing technologies have upended basic biology, providing researchers the ability to rewrite the genetic material inside living cells. However, the earliest versions of CRISPR only worked in some cell types and weren’t ready for use in humans—the technology often had low yields, successfully changing the genes in only a small number of treated cells.
Researchers at Gladstone—including Jennifer Doudna, PhD, who received a Nobel Prize for the discovery and development of CRISPR genome editing technology—have pushed the envelope on these technologies, debuting more precise ways of editing genes and applying the approaches to immune cells. In 2015, Doudna and Marson reported that they had successfully edited human T cells (a type of immune cell that can fight infections and cancer) with CRISPR, setting up the technology for use in immunology research.
At the time, the researchers could only edit a few nucleotides (the basic building blocks of DNA) at a time. Now, the possibilities are far vaster; they can re-write thousands of nucleotides at once. They can also carry out experiments where they simultaneously probe the effects of thousands of genomic edits on immune cells at once. And, in a step toward new immune-based therapies, they can make precise edits that are compatible with clinical manufacturing in terms of process and yield.
Revealing Immune Cell Wiring
In 2018, Marson’s group described a powerful new method for genome-wide CRISPR-based screens to reveal how immune cells are wired—key insights for repurposing the cells as therapeutics. This early study allowed them to identify and better understand genes that could be disrupted to improve the function of T cells designed to fight cancer.
This type of massive, high-throughput CRISPR screen to discover genes with important roles in immune cells is becoming easier, faster, and more common. Researchers at the Gladstone-UCSF Institute of Genomic Immunology are continuously spearheading new versions of the screens—some that use CRISPR to remove new types of genes and genetic elements, and others that use entirely new versions of the CRISPR technology.

Marson and his team are working to better understand how to edit immune cells so they can be used to fight diseases more effectively than ever before.
Marson’s group, for instance, used CRISPR to disrupt the genes for thousands of transcription factors—master genes that control the activation of many other genes—and identify which of them altered three genes associated with immune disease in T cells. The results highlighted just how interconnected gene activation networks are; many of the same genes not only regulated the three central molecules, but each other at the same time.
The researchers also became the first to use CRISPR across the entire genome to activate (instead of disrupt) genes in human immune cells, using a tool known as CRISPRa. They used the approach to control the activation of nearly 20,000 genes in human T cells and homed in on hundreds that regulate T cell activity.
Together, these discoveries are building a detailed map of the genes that give our immune system its ability to eliminate pathogens and foreign cells. This map, the researchers say, will help them edit our immune cells so that that they can fight disease more effectively than ever before.
“When we combine the power of single-cell genomics with high-throughput studies using CRISPR, we can really start understanding the basic rulebook of how genes are controlling immune cells,” says Marson. “Now, we’re taking these lessons and using them to actually start reprogramming human immune cells to have new functions.”
Understanding Cancer Immunology
Although Marson and his colleagues aspire to use reprogrammed immune cells to treat a variety of diseases, one of the clearest applications is in cancer, which has already been shown to be susceptible to attack by the immune system.
Kole Roybal, PhD, an associate professor in the Department of Microbiology and Immunology at UCSF and an affiliate investigator at Gladstone, works on designing new and improved receptors that direct engineered T cells to recognize and kill solid tumors.

Roybal (center) is hoping to improve cancer treatments by designing new ways for engineered T cells to recognize and kill solid tumors.
In April 2022, his team described a new set of receptors that can be put into T cells and fine-tuned to not only kill cancer cells, but deliver other molecules to tumors. The group used the engineered cells in mouse models of leukemia, mesothelioma, and ovarian cancer and showed that they eliminated cancer cells effectively and selectively.
Roybal also co-founded a company with Marson, Arsenal Bio, which is working on additional cell therapies using many different versions of these receptors programmed into immune cells via CRISPR.
To optimize the use of engineered immune cells to treat cancer, however, researchers also need a better understanding of solid tumors—how cancer cells organize themselves and produce molecules to block the immune system, and what factors make a more effective immune response against these tumors. Cancer immunologist Karin Pelka, PhD, who joined Gladstone as an assistant investigator in 2021, tackles such questions.
Last year, while still at the Broad Institute of MIT and Harvard, Pelka discovered specific clusters of immune cells inside a subset of colorectal tumors. The unique conformations of the cells—which her team calls “immune hubs”—helped the immune system recognize cancers in these patients. She is now continuing work on how the various cells that come together in immune hubs communicate with each other, and how these interactions can be controlled to make immunotherapies more effective.

Pelka is studying how interactions between immune cells can be controlled to make immunotherapies more effective.
“We are trying to understand which types of hubs are relevant to therapeutic outcomes,” Pelka said at a virtual Innovators of Genomic Immunology meeting held by Gladstone in November 2021. “We’re now comparing patient specimens from before and after treatment to see what features we have to modulate to get immunotherapy to work.”
Recent technological advances are helping researchers like Pelka understand the distribution of different kinds of cells within tissues and tumors, and create maps at the single-cell level.
Last year, Marson’s group, in collaboration with Jimmie Ye, PhD, associate professor of medicine at UCSF and affiliate investigator at Gladstone, published a new technique that does just this. Called XYZeq, it provides a map of a tumor by dividing it into hundreds of small areas, so researchers can see which types of cells neighbor which in the tissue, and what genes they turn on or off.
“When we have single-cell sequencing data that includes spatial information, we can start asking questions about the types of cells that are adjacent to each other and interacting in healthy and diseased tissues,” says Ye.
Keeping T Cells Active
One of the long-standing challenges in launching immune responses against tumors is that over time, these therapies tend to stop working. When T cell receptors are constantly activated, the T cells gradually enter a state of dysfunction known as T cell exhaustion, in which they produce fewer immune molecules and become less effective at destroying cancer cells or pathogens. Overcoming this exhaustion is critical for researchers trying to use T cells therapeutically.
Recently, two Gladstone affiliate investigators—Ansuman Satpathy, MD, PhD, an assistant professor in the Department of Pathology at the Stanford School of Medicine, and Julia Carnevale, MD, an assistant professor of hematology and oncology at UCSF—used CRISPR to alter tens of thousands of genes in T cells and then tested which cells showed signs of exhaustion. They pinpointed dozens of genes, many of them epigenetic factors that simultaneously control the expression of numerous other genes.
“We want to push the boundaries of what’s possible. It’s an ambitious goal, but it’s within reach because of the incredible team we’ve assembled.”
When Satpathy’s team engineered T cells to lack one of these epigenetic genes, known as Arid1a, levels of T cells increased and showed molecular signs of being healthier and more persistent.
In another study, Carnevale used CRISPR to alter another gene of interest—RASA2—in T cells. Without the gene, she and her colleagues found, T cells were less likely to become exhausted.
“T cells engineered to lack RASA2 could just keep killing,” says Carnevale. “It seems as if we found a brake in the system and when we take it off, we unleash the potential of these therapeutic cells.”
T Cell Therapy Beyond Cancer
Treating cancer is one of the clearest and most immediate applications for engineered T cells, but researchers at the Gladstone-UCSF Institute of Genomic Immunology are also studying living T cell therapies for other applications. Qizhi Tang, PhD, professor of surgery at UCSF and affiliate investigator at Gladstone, is leading clinical trials to test regulatory T cell therapy in type 1 diabetes patients and organ transplant recipients.
Transplant recipients must take immunosuppressive drugs to prevent their bodies from rejecting their new organs. Animal models have suggested that a type of T cells known as regulatory T cells may be a safe, long-lasting way to prevent this rejection. Tang and her colleagues have carried out safety and feasibility trials of the therapy in transplant patients.
In November 2022, her team reported a Phase II study testing the efficacy of the therapy in a small group of liver transplant patients. The study found that regulatory T cells are lost after liver transplantation. Now, her lab is studying cell engineering strategies to make regulatory T cells work better and last longer.

Investigators in the Gladstone-UCSF Institute of Genomic Immunology are striving to transform medicine through living therapeutics. Back row, left to right: Kole Roybal, Alex Marson, Ansuman Satpathy, Stacie Dodgson, Justin Eyquem. Front row, left to right: Qizhi Tang, Matthew Spitzer, Jimmie Ye, Karin Pelka, Julia Carnevale, Bruce Schaar (former institute member).
Marson’s group is also interested in better understanding the wiring of regulatory T cells, which act as an immune system brake to prevent inflammation, with the goal of facilitating their use as therapeutics for autoimmune diseases.
He and his collaborators used CRISPR technology to remove 40 different transcription factors from regulatory T cells. Then, the researchers used high-throughput gene sequencing to study how tens of thousands of other genes were impacted. The results revealed the genetic programs that give regulatory T cells their ability to control immune responses.
“This kind of insight will help researchers learn how to tweak genetic programs in regulatory T cells to work toward therapeutic benefit in a wide range of human diseases,” says Marson.
Scaling Cell Manufacturing
One of the major challenges to the nascent field of cellular therapy is improving cell manufacturing protocols.
“In the future, we need more robust cell manufacturing technologies,” says Tang. “Gene editing must be high-yield and safe; cells must be affordable and easy to manufacture by the billions.”
“When we start thinking about making clinical products, a key step is scaling up production, which can be extremely expensive with current methodologies,” agrees Brian Shy, MD, PhD, of the UCSF Experimental Cellular Therapy Group, who works with Marson on the translational aspect of manufacturing living therapies.
One of the key tenets of the Gladstone-UCSF Institute of Genomic Immunology is that researchers are collaboratively working on all these issues at the same time—they’re not waiting to perfect one element before moving to the next.

Marson's goal for the Gladstone-UCSF Institute of Genomic Immunology is for scientists with different expertise to collaborate and fuel innovation.
“We have this iterative circle within our ecosystem where researchers are driving a better understanding of human immune cells, clinicians are weighing in on the research and ensuring that the work has direct applications, and other teams are improving the way we make the cells,” says Stacie Dodgson, PhD, the institute’s scientific strategy manager.
In one recent paper, Marson and Shy debuted a new gene editing technology to introduce large therapeutic constructs into cells along with the CRISPR machinery. Compared to prior approaches, this new technology can handle much longer strands of DNA and works more efficiently to edit cells. These improvements make it easier to produce massive amounts of CAR-T cells, says Shy.
“We showed that we were able to make more than one billion cells in a single run, which is well beyond the range of cells we need for clinical trials,” he says.
A Path Forward
Marson is the first to admit that his dream of transforming medicine through living therapeutics is a lofty one. But he doesn’t think it’s out of reach. With the progress that he and his colleagues have made in just a few years, they are already leaps and bounds closer to clinical applications.
“We want to push the boundaries of what’s possible,” Marson says. “The Institute of Genomic Immunology is built to take ideas and apply them through preclinical models, cell manufacturing, and all the way into the clinic. It’s an ambitious goal, but it’s within reach because of the incredible team we’ve assembled.”
Today, the institute’s infrastructure to foster deep collaboration and new ideas has been established. And the science will keep coming.
“The goal here is too big for just one lab to tackle, but there’s huge enthusiasm to create neighborhoods where we can work together and encourage scientists with different expertise to collaborate and fuel innovation,” Marson says. “It requires long-term trust and a collaborative spirit. We have all that here.”
Featured Experts
Support Discovery Science
Your gift to Gladstone will allow our researchers to pursue high-quality science, focus on disease, and train the next generation of scientific thought leaders.
Gladstone’s Scientific Highlights of 2025
Gladstone’s Scientific Highlights of 2025
From fundamental insights to translational advances, here’s how Gladstone researchers moved science forward in 2025.
Gladstone Experts Alzheimer’s Disease Autoimmune Diseases COVID-19 Neurological Disease Genomic Immunology Cardiovascular Disease Data Science and Biotechnology Infectious Disease Conklin LabScience in Seconds | Researchers Pinpoint Key Gene Behind Heart Defects in Down Syndrome
Science in Seconds | Researchers Pinpoint Key Gene Behind Heart Defects in Down Syndrome
In this video, Gladstone scientists share how they used stem cells, gene editing, and AI to identify a gene driving heart defects in Down syndrome—and how reducing its levels in mice restored normal heart development, offering hope for future treatments
Gladstone Experts Cardiovascular Disease Data Science and Biotechnology Pollard Lab Srivastava Lab AI Big Data CRISPR/Gene Editing Human Genetics Stem Cells/iPSCsScience in Seconds | The Thinking Microscope: Research Powered by an AI Brain
Science in Seconds | The Thinking Microscope: Research Powered by an AI Brain
In this video, Steve Finkbeiner and Jeremy Linsley showcase Gladstone’s groundbreaking “thinking microscope”—an AI-powered system that can design, conduct, and analyze experiments autonomously to uncover new insights into diseases like Alzheimer’s, Parkinson’s, and ALS.
Gladstone Experts ALS Alzheimer’s Disease Parkinson’s Disease Neurological Disease Finkbeiner Lab AI Big Data









