Gladstone NOW: The Campaign Join Us on the Journey✕
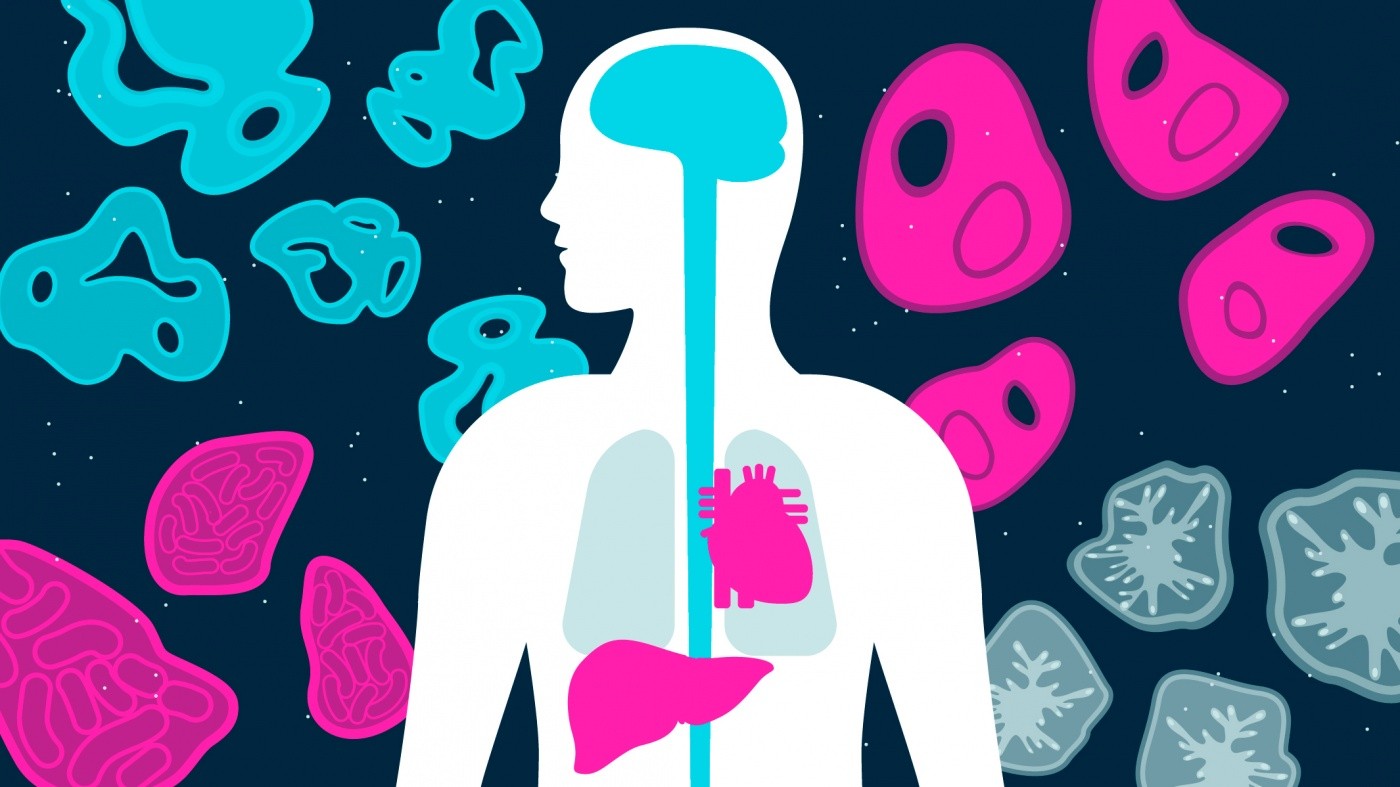
Organoids consist of multiple cell types organized into 3-D structures that resemble the features and functions of real human organs.
When the phone call comes, it’s all hands on deck in Melanie Ott’s lab. A patient with hepatitis C is going in for surgery to remove a liver tumor, and the research team must prepare to receive a sample of the liver cells.
With the patient’s permission, the cells are rushed across town (freezing them would ruin them) from the operating room to the lab. There, the researchers quickly yet carefully spread and suspend them within a nutritious gel. Then, they wait.
One week later, just evident to the naked eye, the “seed” cells—stem cells found in the patient’s liver—have multiplied, diversified, and grown into miniature versions of the organ. Each consisting of about 300 cells, and about as wide as a strand of hair, these mini-livers hold clues to the mechanisms of hepatitis C infection.
“Studying them is deepening our understanding of the virus and could open new paths to treatment,” said Senior Investigator Melanie Ott, MD, PhD.
Her lab’s mini-livers are just one of several types of miniature organ-like structures, commonly called organoids, grown at Gladstone Institutes. These tiny versions of the heart, brain, intestine, lungs, and other organs are transforming how we research human disease and development at Gladstone and beyond.
Organoids consist of multiple cell types organized into 3-D structures that resemble the features and functions of real human organs. Often surprisingly self-regulating, they develop naturally according to instructions encoded in the genetic material of the stem cells from which they grow.
“Certain aspects of human biology are too distinct to be modeled in animals, and typical 2-D cell cultures bear little resemblance to the 3-D physiology of the human body,” said Senior Investigator Todd McDevitt, PhD. “Organoids open a new frontier for discovery.”
Engineering Organoids to Study Human Development
McDevitt’s lab has been studying organoids for 15 years. Early on, his team made key advancements that paved the way for today’s gold rush of organoid research. For instance, they found that gently shaking 3-D cell cultures prevents them from forming unmanageable clumps.
“Shaking organoids allows them to be cultured for long periods of time,” McDevitt said. “We’ve had some go as long as 2 years now.”
Now, he continues to lead the field. He is engineering new ways to grow and monitor activity in organoids, and driving new insights into human development.
Earlier this year, starting with stem cells derived from adult human cells, McDevitt’s team has worked out how to grow 3-D clusters containing key cells that arise during development of the spinal cord. These particular cells are found in the human hindbrain, and equivalent cells in mice are known to generate signals that help regulate breathing.
Sure enough, when McDevitt’s team measured the activity of the cells they grew, they saw the same signaling patterns typically observed in the equivalent mouse cells, meaning they had successfully reproduced a vital aspect of hindbrain development.
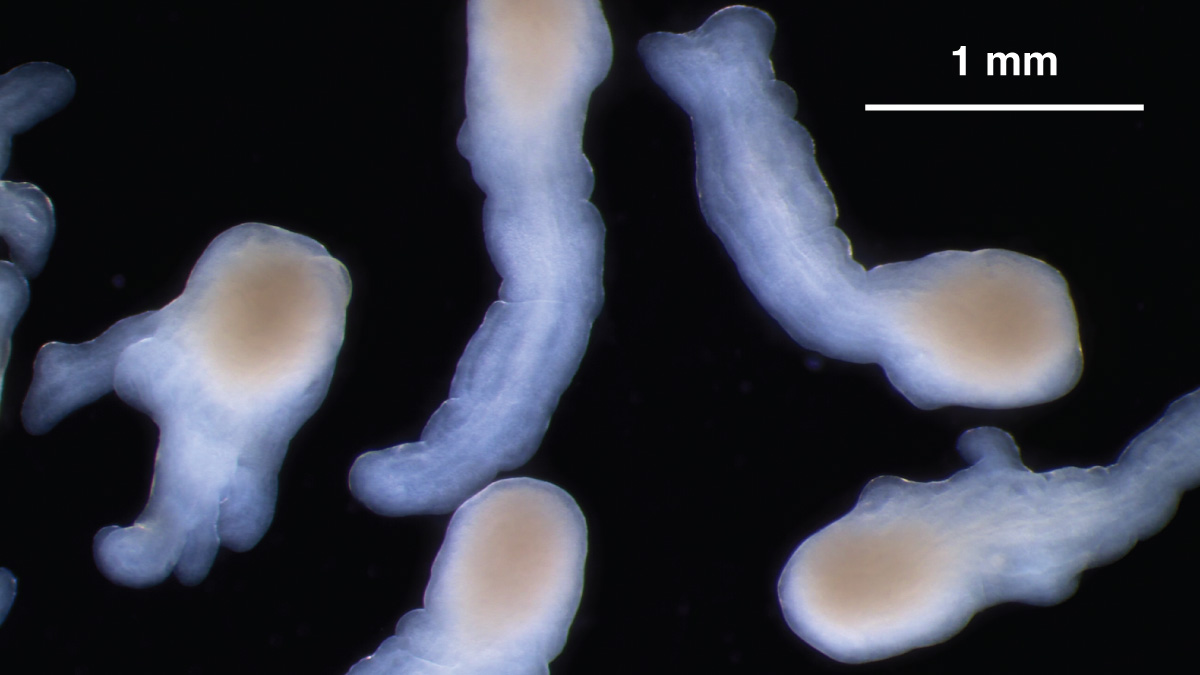
Under the right culture conditions, organoids derived from hiPS cells can mimic the elongation of a normal spinal cord. Credit: Nick Elder, McDevitt Lab, Gladstone Institutes
More recently, also working with hindbrain cultures, a small team of his bioengineering and developmental biology students found they could alter a few key parameters to make the structures elongate instead of maintaining their usual spherical shape. This elongation is consistent with the process by which the human spinal cord develops.
“It’s amazing. I’m still in shock every time I see it,” McDevitt said.
McDevitt is careful to distinguish between true organoids and other 3-D cultures called microtissues. Microtissues are less representative of actual organ structure, but serve as powerful platforms for studying human tissues and disease.
The precise criteria for labeling a 3-D cell structure an organoid versus a microtissue are still under debate. Still, even by the strictest definition, McDevitt believes his lab may have created the first true spinal cord neural tube organoid.
Viruses in the Brain, and Beyond
Warner Greene, MD, PhD, is also interested in neural organoids, but for a very different reason. As director of the Center for HIV Cure Research, he is kicking off a project using them to study reservoirs of HIV found in the brains of people with the virus.
“HIV in the brain is relatively difficult to study,” Greene said. “But we cannot neglect it, because the brain can re-trigger an infection even if we have a perfect cure for the rest of the body.”
Working with McDevitt and former Gladstone Senior Investigator Li Gan, PhD, he will use organoids containing multiple kinds of brain cells to investigate longstanding mysteries about the mechanisms of HIV brain infection. He will also test a potential strategy to permanently deactivate the virus in the brain, which could one day be used in HIV patients.
Ott has been growing organoids from HIV patients’ intestinal cells and co-culturing them alongside immune cells.
“HIV doesn’t actually infect the gut, but it infects key immune cells, and this in turn results in gut dysfunction,” Ott said. “We’re studying what the virus is actually doing in this context, in a much more feasible and direct way than we could in mice or monkeys.”
Beyond HIV, she is applying a similar technique to study the role of immune cells in other viral infections, using different kinds of organoids. For instance, she is growing lung organoids to explore how the cells contribute to influenza infection.
“Immune mechanisms are responsible for many of the dangerous symptoms of viral infections, so understanding them could reveal new opportunities to target them for treatment,” she said.
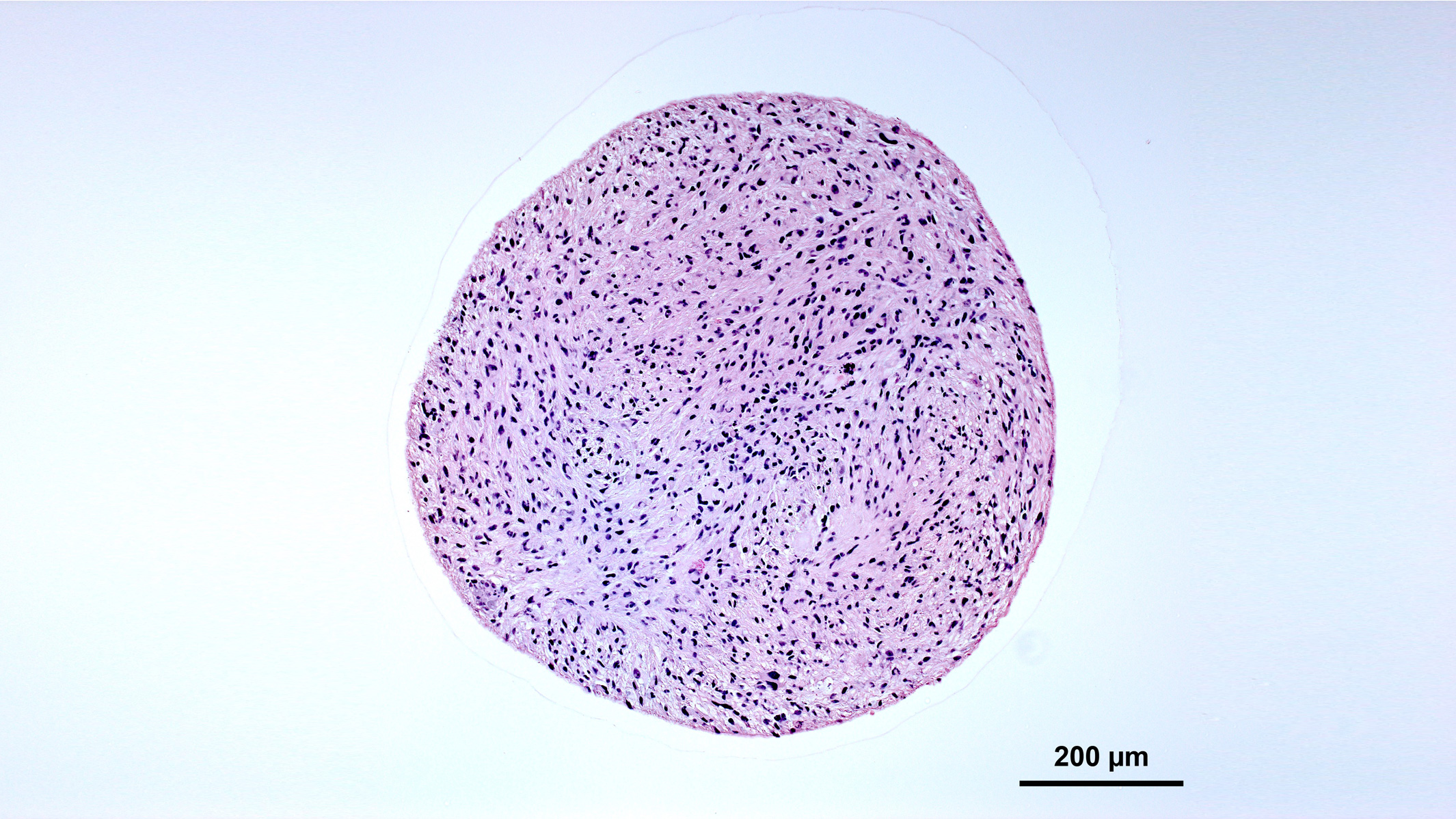
Organoids often remain as spherical balls of cells. Credit: Nick Elder, McDevitt Lab, Gladstone Institutes
A “Remarkable” Marriage of Technologies
When it comes to genetic disease, organoids and microtissues offer an innovative platform for personalized medicine. Senior Investigator Bruce Conklin, MD, is using microtissues to study diseases of motor neurons, which extend from the spinal cord to muscles in the hands and feet.
Like Ott, Conklin uses progenitor cells voluntarily provided by patients as “seeds” to grow 3-D motor-neuron cell cultures in his lab. Each patient has a distinct set of genetic mutations underlying their motor-neuron disease, and these mutations persist in the DNA of the cultures grown from their donated cells.
“Microtissues and organoids now give us access to basically everything we could want for genome editing.”
By strict definition, Conklin’s 3-D motor-neuron cultures are more accurately called microtissues than organoids, since they are less representative of actual organ function. Still, they are an ideal platform for Conklin’s work.
Here, microtissues meet genome-editing technology. Conklin is using the motor neuron microtissues as a safe but realistic setting to experiment on human DNA with a tool called CRISPR. He wants to see if he can use CRISPR to edit individual patients’ genes and thereby fix their disease-causing mutations.
“Once we can do this in microtissues, we’ll know it works, and then it will just become a matter of how to deliver the editing materials to patients,” Conklin said. “Our goal is to potentially alter the course of disease, or even provide a cure.”
To develop this technique, he is starting out with a more predictable condition known as Charcot-Marie-Tooth (CMT) disease. Later, he hopes the findings can also benefit people with more complex motor neuron diseases, such as amyotrophic lateral sclerosis (ALS).
“Microtissues and organoids now give us access to basically everything we could want for genome editing,” Conklin said. “It’s a remarkable marriage between two technologies that are really very powerful.”
Single-Cell Resolution
Another recent technology that enhances organoid research is single-cell RNA profiling. This tool enables researchers to pinpoint gene expression in the individual cell types that make up an organoid, providing “a resolution that is really unprecedented,” Ott said.
She and Greene are each using single-cell RNA profiling to get a high-resolution view of the mechanisms of viral infection.
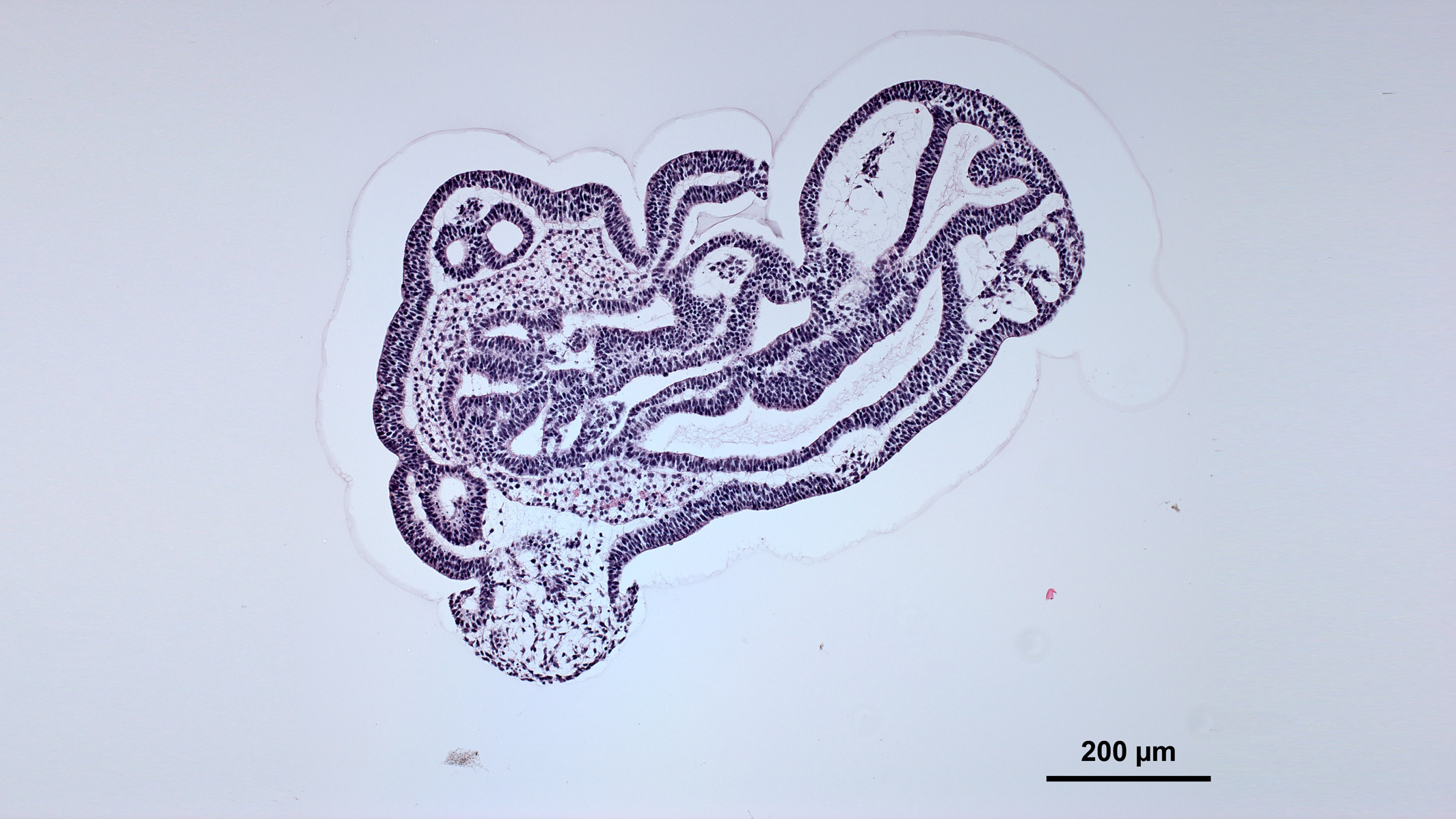
Sometimes, organoids display cell layers and structural features reminiscent of actual tissues. Credit: Nick Elder, McDevitt Lab, Gladstone Institutes
McDevitt is also using the technique in ongoing research on microtissues that mimic human cardiac tissue. Instead of growing self-assembling mini-hearts, this project uses a hands-on approach to combine cell types that are not actually found together in human tissues.
“We’re breaking and inverting normal cellular networks to see if we can learn some biological rules,” McDevitt said. By analyzing gene expression of individual cells in these mix-and-match tissues, he can see how they influence each other, gaining new insights into how different cell types contribute to tissue function as a whole.
McDevitt and his Gladstone colleagues continue to push the boundaries of organoid and microtissue research, paving the way to unprecedented understanding of human biology and the potential for innovative medical treatments.
“I’m very happy that Gladstone made early, painstaking investments in organoid research,” Ott said. “Now we have so many opportunities to move ahead and help other researchers as we work toward translating our findings in meaningful ways for patients.”
Support Discovery Science
Your gift to Gladstone will allow our researchers to pursue high-quality science, focus on disease, and train the next generation of scientific thought leaders.
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Gladstone scientists identified a cellular boundary that guides heart development and revealed how disrupting it can lead to holes in the heart’s wall.
News Release Research (Publication) Congenital Heart Disease Cardiovascular Disease Bruneau LabGene Editing Strategy Could Treat Hundreds of Inherited Diseases More Effectively
Gene Editing Strategy Could Treat Hundreds of Inherited Diseases More Effectively
Scientists at Gladstone show the new method could treat the majority of patients with Charcot-Marie-Tooth disease.
News Release Research (Publication) Neurological Disease Conklin Lab CRISPR/Gene EditingGenomic Maps Untangle the Complex Roots of Disease
Genomic Maps Untangle the Complex Roots of Disease
Findings of the new study in Nature could streamline scientific discovery and accelerate drug development.
News Release Research (Publication) Marson Lab Genomics Genomic Immunology



