Gladstone NOW: The Campaign Join Us on the Journey✕

Sukrit Silas joined Gladstone Institutes in August 2024. His lab focuses on leveraging the therapeutic power of bacteriophages—more commonly known as “phages”—to overcome antimicrobial resistance.
Antimicrobial resistance is on the rise—and the consequences are deadly. More than 39 million people around the world could die from drug-resistant infections over the next 25 years, according to new analysis by the Global Research on Antimicrobial Resistance Project. It’s a crisis that puts many gains of modern medicine at risk.
In response, scientists are stepping up efforts to develop a new generation of antimicrobial drugs. One of those scientists is Sukrit Silas, PhD, assistant investigator at Gladstone Institutes, whose research is focused on harnessing the therapeutic power of bacteriophages—more commonly known as “phages.”
Phages are viruses that naturally infect and often kill bacteria in our bodies and throughout all of Earth’s ecosystems, making them a promising alternative for treating infections. Silas, who joined Gladstone in August, will carry out large-scale screens of tens of thousands of phage genes in wild bacterial strains to identify those with the best potential to counter today’s top antimicrobial threats.
“Genetic diversity in the phage world is mind-bogglingly huge,” Silas says. “But today, we have the tools to make scientific breakthroughs that take phages from a basic biology problem to a viable therapeutic.”
Indeed, phages are recognized as the world’s most abundant biological agent. But until recently, research has mainly focused on the interplay between just a handful of phages and a few lab strains of bacteria. While this has led to revolutionary breakthroughs—including new tools for genetic engineering, such as CRISPR—the broader world of phage-based antimicrobial strategies and bacterial counterstrategies has been overlooked, Silas says.
“Genetic diversity in the phage world is mind-bogglingly huge. Today, we have the tools to make scientific breakthroughs that take phages from a basic biology problem to a viable therapeutic.”
He’s poised to capture missed knowledge using high-throughput screening methods to look beyond the phage genes that are typically studied. Already, he has found critical barriers that these viruses must overcome to enter bacterial cells—knowledge that will help in designing new antimicrobial therapies.
Silas opened his lab, part of the Gladstone Institute of Virology, after completing his postdoctoral work in the UC San Francisco (UCSF) lab of Joe Bondy-Denomy, PhD, where he served as a Damon Runyon Fellow.
“The phage expertise that Sukrit brings to Gladstone allows us to expand our work into viruses that infect bacteria,” says Melanie Ott, MD, PhD, director of the Gladstone Institute of Virology. “This is a whole field focused on understanding how viruses affect our health by manipulating the bacteria controlling us. Everyone knows about the microbiome, yet we have little understanding of the importance of the virome—the vast collection of viruses in and on our body. Sukrit will play a major role in expanding what we know and how we can use this knowledge to improve global health.”
Pushing Into New Frontiers
Silas can’t remember a time when he didn’t want to be a scientist. As a child growing up in India’s capital city of Delhi, he would pose scientific questions that no one in his family could answer. In high school, Silas stumped his teachers by asking how long a molecule of human DNA could get (the answer: chromosome 1 is the largest, at a half-foot in length).
He finally had the opportunity to quench his curiosity as an undergraduate at Princeton University, working in the lab of Eric Wieschaus, PhD, a 1995 Nobel Prize winner for discovering genetic controls of early embryonic development. Silas would stay at the lab through the night just to have unfettered access to the microscope and other equipment for his research into the cytoskeleton of the Drosophila fruit fly embryo.
The experience confirmed his love of scientific inquiry and taught him something else that would guide his career: “I’m most comfortable in places where very little is known,” Silas says. “Then and now, I’m drawn to new frontiers.”
As he began his doctorate program at Stanford University in the lab of Andrew Fire, PhD, that new frontier was CRISPR—now a widely used gene editing technology, but then a newly discovered immune system used by bacteria and archaea (micro-organisms similar to bacteria).
Silas recalls spending hours discussing the budding research field with fellow lab members and Fire, a 2006 Nobel laureate for his work unveiling a phenomenon known as RNA interference. Silas ultimately dove in with research of his own, discovering a CRISPR adaptation pathway that allows bacteria to potentially defend themselves against RNA viruses.
“The CRISPR world is where I cut my teeth in science,” he says.
The work inspired Silas to lead a study that took stock of RNA viruses in the ocean, greatly expanding awareness of the diversity of RNA viruses in the environment.
A Taste of the Biotech Industry
Ever since the earliest days of his research career, Silas expected his work would always take place in an academic setting. But in the late 2010s, he briefly detoured into the biotech industry to commercialize a non-invasive prenatal screening test based on a method he previously developed.
Silas cofounded a biotech company called BillionToOne and oversaw two clinical trials of diagnostic tests for inherited, single-gene diseases such as beta thalassemia and sickle cell anemia. The trials took place in India, where there’s a great need for prenatal screening for these diseases. His company demonstrated the tests worked, leading to their marketing in the US and parts of Europe. Silas hopes they’ll eventually be available in India, as well.
“Co-founding a biotech company was one of the most useful experiences of my life. But once the trials were finished, I had to make a choice. I decided to come back to basic science.”
In 2019, Forbes included Silas on its “30 Under 30” healthcare list for his work with BillionToOne.
“Co-founding a biotech company was one of the most useful experiences of my life,” Silas says. “But once the trials were finished, I had to make a choice. I could spend the next 10 years learning diagnostics and business, or I could follow the science wherever it leads me next. I decided to come back to basic science.”
Silas left his company in the capable hands of his co-founders and headed to the lab of Jonathan Weissman, PhD, then at UCSF. Silas returned to his research on bacterial immunity and viruses, but took a new approach involving high-throughput screening—a specialty of the Weissman Lab.
As it turned out, Silas only had about a year to learn the lab’s unique screening approaches before Weissman moved his lab across the country to the Massachusetts Institute of Technology. Not wanting to uproot, Silas found his new research home in Bondy-Denomy’s UCSF lab, where he spent several years before joining Gladstone.
Discovering New Antimicrobial Strategies
Though Silas chose to leave the biotech industry, his work there shaped his approach to his postdoc years. Inspired by seeing an idea come to fruition and make an impact in the real world, he sought out new projects with the potential to address diseases of unmet clinical need, particularly in vulnerable populations.
“I really think of the postdoc experience as an incubator where you can try something risky,” Silas says. “It’s not just a step on the career ladder.”
The Bondy-Denomy lab was just starting to explore “accessory genes” in phages. Unlike genes required for the replication or structural elements of the phage, accessory genes are deemed non-essential: sometimes present, sometimes absent even in closely related variants of the same phage. As such, they were long believed to be relatively inconsequential.
“It’s a whole class of genes that are often overlooked,” Silas says.
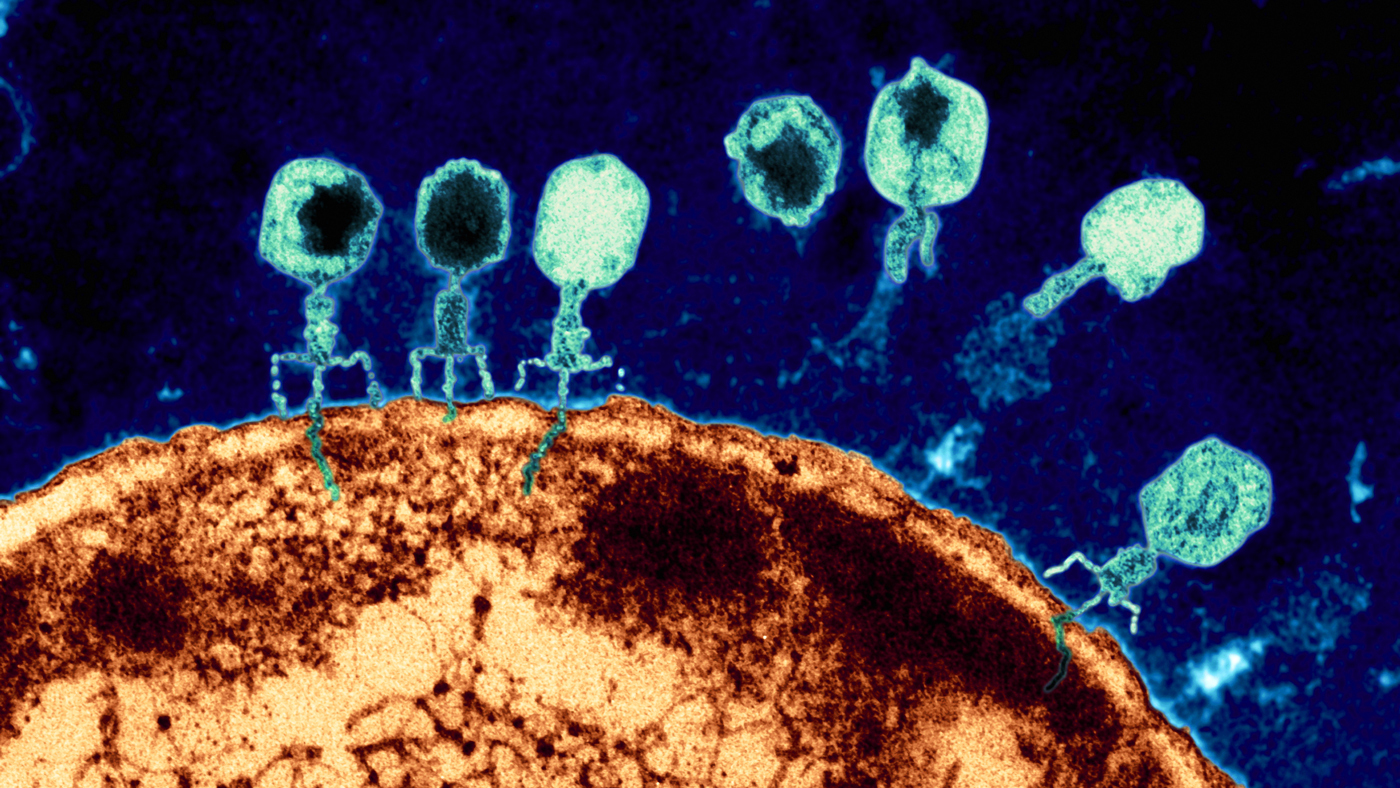
A magnified view of phages attacking an E. coli bacterial cell. Phages, abundant throughout nature, offer an alternative approach to fighting infections that no longer respond to traditional antibiotics. (Image credit: Eye of Science)
Curious about what these genes actually do, Silas embarked on a project to comprehensively identify accessory genes in phages of Enterobacteria. He developed an algorithm that led him to find more than 20,000 of them across E. coli, Salmonella, Klebsiella, and other Enterobacteria. He then built a screening platform to rapidly characterize thousands of accessory genes and how they shape the outcomes of phage infections in wild bacteria.
Through this, he made the surprising discovery that the same accessory genes that disable defenses in some strains of bacteria can activate protective mechanisms in others. He also found that several of the accessory genes could modify the long sugary molecules on the surface of bacterial cells, thereby changing which phages could and couldn’t gain access to the cell.
“We moved from thinking all about immune systems in bacteria to realizing that immune systems are the second layer of defense. The first layer is the cell surface,” Silas says.
At Gladstone, Silas is scaling up the platform he designed to screen each of the 20,000 accessory genes in individual cells of various wild strains of Enterobacteria, and test their ability to modulate infection by diverse families of phages. The work aims to find entirely new antimicrobial properties and discover new strategies for changing the bacterial cell surface.
“Phages hold great potential to address the urgent need for successful medicines.”
“Determining the core bacterial pathways and the cell surface proteins targeted by phage accessory genes will require high-throughput proteomics that you could only do at a few places in the world, and Gladstone is one of them,” Silas says. “This could enhance our understanding not only of phage strategies to remodel the bacterial cell surface, but the basic biology of the outer cell membrane. It will be incredibly exciting to deploy this platform in mycobacteria, as well.”
The project started as a collaboration that also included Silas’ students Héloïse Carion and David Sanchez-Godinez (formerly of the Bondy-Denomy lab), Gladstone Senior Investigator Nevan Krogan, PhD, members of the Krogan lab, and Danielle Swaney, PhD, a Gladstone visiting scientist. Silas plans to continue the collaborations at Gladstone.
Ultimately, Silas intends to reveal an array of phage-based antimicrobial strategies and learn more about the counterstrategies that bacteria use to resist antibiotics.
“Is there an overall logic to bacterial immune systems and how they are organized into a defensive architecture, or does the logic change from species to species and niche to niche?” he asks.
Answering this question will advance the development of phage-based therapies and uncover how much the therapies will need to be customized based on the bacterial strain and other factors. The work also may predict how bacterial resistance evolves.
It’s an ambitious goal, but Silas knows his work is critical to addressing a growing problem that affects all regions of the world and cuts across all income levels—a problem that the World Health Organization classifies as a top global health threat.
The crisis of drug-resistant pathogens—which arose largely out of overuse or misuse of antibiotics in medicine and agriculture—is compounded by the fact that the world faces a dwindling antimicrobials pipeline, with inadequate research and development into new medicines.
“Phages hold great potential to address the urgent need for successful medicines,” Silas says. “We have much more work ahead of us, but I’m hopeful we can begin to create new strategies for defeating infections that threaten lives all over the world.”
For Media
Kelly Quigley
Director, Science Communications and Media Relations
415.734.2690
Email
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Featured Experts
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Gladstone scientists identified a cellular boundary that guides heart development and revealed how disrupting it can lead to holes in the heart’s wall.
News Release Research (Publication) Congenital Heart Disease Cardiovascular Disease Bruneau LabGene Editing Strategy Could Treat Hundreds of Inherited Diseases More Effectively
Gene Editing Strategy Could Treat Hundreds of Inherited Diseases More Effectively
Scientists at Gladstone show the new method could treat the majority of patients with Charcot-Marie-Tooth disease.
News Release Research (Publication) Neurological Disease Conklin Lab CRISPR/Gene EditingGenomic Maps Untangle the Complex Roots of Disease
Genomic Maps Untangle the Complex Roots of Disease
Findings of the new study in Nature could streamline scientific discovery and accelerate drug development.
News Release Research (Publication) Marson Lab Genomics Genomic Immunology