Gladstone NOW: The Campaign Join Us on the Journey✕

A team of scientists led by Deepak Srivastava (left), Arun Padmanabhan (center), and Michael Alexanian (right) uncovered how immune cells trigger a chain of events that cause fibrosis in the heart. Their findings could lead to the development of immune therapies targeting heart disease and chronic conditions in other organs.
If you cut your arm or undergo surgery, scarring can be a good thing; the scar tissue produced by cells called fibroblasts helps you heal. In most organs of the body, however, the accumulation of scarring (called fibrosis) is a sign of chronic disease and aging. Slowing or stopping fibrosis can help treat heart, liver, kidney, and lung diseases. Yet fibrosis of these organs remains a deadly disease with limited treatments.
Now, scientists at Gladstone Institutes have worked out exactly how immune cells trigger scar-forming fibroblasts to become activated and cause fibrosis in the heart, and likely in other organs as well. Blocking the molecular signals between inflammation-causing immune cells and fibroblasts, they showed, can prevent this fibrosis. And, by chance, some of the signals are already targeted by existing drugs, which could potentially be repurposed for heart disease.
“The crosstalk between the immune system and fibroblasts is even more complex than we expected,” says Gladstone Assistant Investigator Michael Alexanian, PhD, the corresponding author of the new study published in Nature. “But now that we’ve begun to map it out, we may be able to develop therapies that target both inflammation and fibrosis.”

Padmanabhan (left), Alexanian (center), and Srivastava (right) are working to understand the precise roles of inflammation and fibrosis in heart failure, which affects 25 million people worldwide.
“Inflammation and fibrosis both play incredibly important roles in the worsening of heart failure, which affects 25 million people worldwide, and many other fibrotic diseases,” adds Deepak Srivastava, MD, president and senior investigator at Gladstone and senior author of the study. “Discovering new ways to stop these processes could help millions of people.”
Heart Disease Changes Both Immune Cells and Fibroblasts
Scientists have long known that both inflammation and fibrosis occur together in diseased organs and with normal aging. Fibrosis in the heart, liver, kidney, and lungs causes unhealthy thickening and stiffening of the organs, which impedes their functions and contributes to organ failure. But the precise role of inflammation, and how it directly causes fibrosis, has not been fully understood.
In the new work, the group used mice to probe how immune cells, in addition to fibroblasts, change during heart disease. They also treated the mice with a drug called a BET inhibitor, which they previously showed prevents fibrosis in animals but has many side effects in other tissues. Their goal was to discern what happens to the cells when heart disease is treated and glean insights that could be used in developing more targeted therapies for humans.
“A number of pro-inflammatory genes were activated in immune cells during heart failure and then repressed during treatment,” says Arun Padmanabhan, MD, PhD, cardiologist, co-first author of the new paper, and former postdoctoral scholar in Srivastava’s lab at Gladstone, who is now an investigator at UC San Francisco (UCSF). “So, it was immediately clear that with this drug, we were shutting down inflammation as well as fibrosis.”
A Signal from Immune Cells to Fibroblasts
Then, through a series of experiments on both the immune cells and fibroblasts, the researchers discovered new molecular connections between inflammation and fibrosis. They detailed a cascade of events that begin with macrophages, immune cells that play a central role in triggering inflammation.
In 2021, the same team had discovered that in fibroblasts, turning on a gene called MEOX1 triggers fibrosis by activating the cells. However, they didn’t know how stress in the heart was sensed by the fibroblasts to became activated.
In the new work, the scientists discovered that when the heart is in a diseased state, the regulatory gene Brd4 is turned on in macrophages, and it makes the immune cells produce IL-1β, a signaling molecule. When it interacts with neighboring fibroblasts, the IL-1β protein activates the “master switch” MEOX1 in fibroblasts—which researchers had already linked to fibrosis.
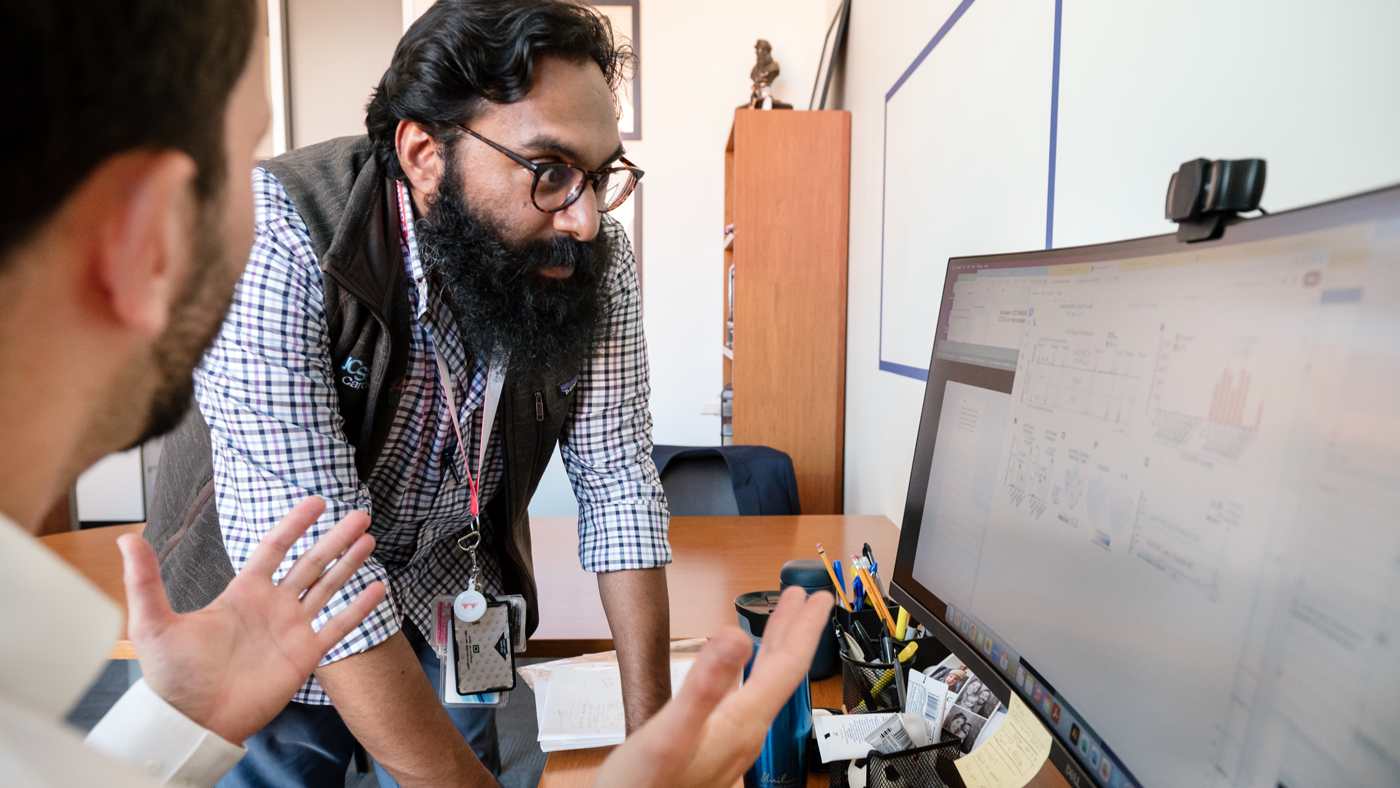
The new study could lead to better ways to prevent fibrosis in patients without blocking the immune system's protective effects. Seen here are Alexanian (left) and Padmanabhan (right) discussing their findings.
“We were able to pinpoint exactly where the inflammation starts in macrophages, and how that affects fibroblasts,” says Padmanabhan. “Understanding this entire chain of events will allow us to intervene earlier in the process from multiple angles and find better ways to prevent fibrosis in patients.”
In fact, the researchers showed that, in mice, blocking different steps of the macrophage-fibrosis pathway—including Brd4 and IL-1β—can help ease heart fibrosis in animals with heart disease.
In addition, because the IL-1β protein has other roles in driving inflammation throughout the body, drugs have already been developed to stop its activity in diseases including cancer, rheumatoid arthritis, and diabetes. But determining whether these drugs influence fibrosis in the heart or other organs will require additional studies.
“Now that we know the signals involved, we can develop targeted drugs that stop fibrosis without blocking the positive effects of the immune system, such as fighting infection,” says Alexanian, who is also a professor in the Department of Pediatrics at UCSF.
For Media
Julie Langelier
Associate Director, Communications
415.734.5000
Email
About the Study
The paper “Chromatin Remodeling Drives Immune Cell–Fibroblast Communication in Heart Failure” was published in the journal Nature on October 23, 2023. Authors are: Michael Alexanian, Arun Padmanabhan, Tomohiro Nishino, Lin Ye, Angelo Pelonero, Clara Youngna Lee, Nandhini Sadagopan, Yu Huang, Kirsten Auclair, Ada Zhu, Yuqian An, Cassandra Martinez, Barbara Gonzalez Teran, Will Flanigan, Charis Kee-Seon Kim, Mauro Costa, Katherine Pollard, Pawel Przytycki, and Deepak Srivastava of Gladstone; Saptarsi Haldar of Amgen; Joshua Travers, Koya Lumbao-Conradson, Ronald Vagnozzi, and Timothy McKinsey of University of Colorado; Christina Ekstrand and Alexis Combes of UC San Francisco; Zachary Gardner, Li Li, and Rajan Jain of University of Pennsylvania; and Israel Charo of ChemoCentryx.
The work was supported by the Swiss National Science Foundation (P400PM_186704), the National Institutes of Health (K08 HL157700, R35HL166663, R01 HL169578, R01 HL116848, R01 HL147558, R01 DK119594, R01 HL150225, K99 HL166708, P01 HL098707, U01 HL098179, P01 HL146366, R01 HL057181, R01 HL015100, R01 HL127240, R01 HL127240), a Longevity Impetus Grant, the Tobacco‐Related Disease Research Program (578649), the A.P. Giannini Foundation (P0527061), the Michael Antonov Charitable Foundation Inc., the Sarnoff Cardiovascular Research Foundation, the Frank. A. Campini Foundation, the Japan Society for the Promotion of Science, the Burroughs Wellcome Fund, the American Heart Association (18POST34080175, 818798, 16SFRN31400013), the San Simeon Fund, the Roddenberry Foundation, the L.K. Whittier Foundation, Dario and Irina Sattui, Younger Family Fund, and Additional Ventures.
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Support Discovery Science
Your gift to Gladstone will allow our researchers to pursue high-quality science, focus on disease, and train the next generation of scientific thought leaders.
New Tool Reveals the Secrets of HIV-Infected Cells
New Tool Reveals the Secrets of HIV-Infected Cells
Developed by Gladstone scientists, HIV-seq could uncover new opportunities for treating HIV.
News Release Research (Publication) HIV/AIDS Infectious Disease Roan LabVitamin B3 Therapy Offers Hope for Fatal Childhood Disease
Vitamin B3 Therapy Offers Hope for Fatal Childhood Disease
A new framework that matches vitamins with genetic diseases helped uncover that high-dose vitamin B3 can dramatically extend survival in mice with NAXD deficiency.
News Release Research (Publication) Rare Diseases Jain LabGladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Gladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Roan has made great strides in understanding how persistent viruses including HIV cause disease and how immunity to viruses shapes human health.
Awards News Release COVID-19 HIV/AIDS Infectious Disease Roan Lab





