Gladstone NOW: The Campaign Join Us on the Journey✕

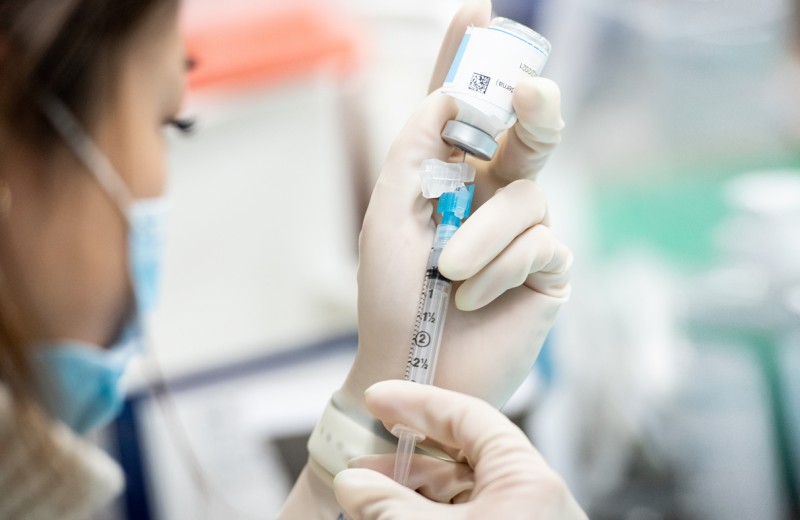
Magnus Hoffmann opened his lab at Gladstone Institutes earlier this year with a mission to create a vaccine platform that provides long-lasting immune protection against a wide range of pathogens.
Magnus Hoffmann, PhD, was about halfway through his doctorate program in biology at the California Institute of Technology when the COVID-19 pandemic hit.
As a graduate student in the lab of renowned Caltech biochemist Pamela Bjorkman, PhD, Hoffmann’s primary interest had been to develop gene therapies to cure HIV.
But as a result of this work, he discovered something else: a novel technology that instructs cells to generate nanoparticles with an uncanny resemblance to an actual virus. These extremely tiny spheres—1,000 times smaller than the width of a human hair—even display a dense array of surface proteins, similar to the spike proteins that dot the surface of the COVID-19 virus.
With vaccines suddenly dominating headlines, Hoffmann began to see how his technology could serve as a platform for developing new vaccines for COVID-19, and potentially other viruses.
“The discovery of the technology was strongly influenced by my prior HIV research, but the initial application was driven by the pandemic,” says Hoffmann, who joined Gladstone Institutes as an assistant investigator in June 2024. “I wanted to show how useful this platform could be, and one of the most obvious ways to do that was applying it to vaccine design. My hope was, and still is, that it will be ready for the next pandemic.”

Hoffmann’s novel vaccine approach repurposes the cellular pathway that some viruses use to bud from infected cells. As a result, it induces the body to create virus-like particles, with spike proteins organized in a dense array around a sphere. These particles look like an actual virus and are readily recognized by the immune system.
As lockdown ensued, Hoffmann refined the technology so it could be easily delivered by genetic material known as mRNA—the same material used for two of the most commonly offered COVID-19 vaccines, from Moderna and Pfizer-BioNTech.
Those vaccines work by instructing the body’s cells to mimic infected cells by producing spike proteins and presenting them on the cell surface. However, Hoffmann’s technology taught cells to do more: produce entire virus-like particles to mimic not only infected cells, but also the virus itself. During a regular viral infection, the immune system would encounter both.
In theory, this would induce a stronger and longer-lasting immune response than other vaccine approaches could provide on their own, while also leveraging mRNA’s fast and scalable manufacturing—critical during a pandemic.
In animal studies, Hoffmann’s technology worked, resulting in antibody responses 10-fold more potent than regular mRNA vaccines against the Omicron variant of SARS-CoV-2, the virus that causes COVID-19. A major study in Cell documents this work, laying the foundation for Hoffmann’s next steps at Gladstone.
“My goal now is to optimize and continue to evaluate this technology,” Hoffmann says.
His Gladstone lab will be working hard to develop different versions of the technology and gain a more complete understanding of the pathogens to which it can be applied.
“We’re very excited to have Magnus on board because he brings complementary expertise to our work in virology, and especially our work with virus-like particles,” says Melanie Ott, MD, PhD, director of the Gladstone Institute of Virology. “He also brings new expertise in vaccines that will truly enrich our research and capabilities.”
Harnessing Cells’ Natural Machinery
The COVID-19 pandemic underscored the importance of vaccines, with nearly 20 million lives saved in the first year of vaccine deployment alone. The commercial rollout of mRNA vaccines, in particular, was a major win, as they could be produced faster and more affordably than traditional vaccines.
But Hoffmann still saw room for improvement.
While the two mRNA vaccines have been highly effective against COVID-19, they require frequent boosters to keep the immune system on guard against a constantly evolving virus.
Later in the pandemic, a more conventional “protein nanoparticle” vaccine emerged. That vaccine, from Novavax, delivers already-made virus-like nanoparticles into the body to activate the immune system. It, too, has been highly effective. However, immune protection still lessens over time and manufacturing is challenging, as nanoparticles must be produced in bioreactors.
With Hoffmann’s technology, the body’s own cells are making the nanoparticles. And because it triggers such a robust response from many different types of immune cells, it may also lead to longer-lasting immunity—reducing the need for boosters.
"Ideally, you want a vaccine that gives you durable immune responses that will last much longer than they do now, and possibly for life.”
Following his study in Cell, Hoffmann’s technology caught the attention of the scientific community. Nature Immunology called it a “two-birds-one-stone” approach, as it mimics infected cells and virus particles—both of which would be present in a natural infection.
“We urgently need new vaccines that can be manufactured rapidly and also elicit long-lasting immune protection,” Hoffmann says. “Even with all the vaccine innovation that emerged during the pandemic, we have important gaps to fill. Ideally, you want a vaccine that gives you durable immune responses that will last much longer than they do now, and possibly for life.”
Mapping the ‘Interactome’
COVID-19 isn’t the only virus that can be targeted by Hoffmann’s technology. He says it could be easily modified to protect against viruses such as influenza and perhaps even HIV. And it has a wide range of other possible applications, including nanoparticle-based drug delivery, which may be useful for cancer and a swath of other diseases.
“Nanoparticles bring a range of benefits for drug delivery,” Hoffmann says.
Chief among those benefits, they’re able to zero-in on targeted cells, delivering their therapeutic “cargo” directly where it’s needed. This maximizes the impact of a medicine while reducing unwanted effects, he says.
In parallel, Hoffmann is pursuing additional research to better understand what shapes our immune response to vaccines. He’s also developing new screening technologies to identify the interactions that occur among receptors on the surface of cells, known collectively as the “interactome.”
“Mapping these interactions is critical because most drugs target receptor proteins, yet for most of these proteins, little is known about how they interact with one another or the cellular environment,” Hoffmann says.
By identifying new interactions that activate important cellular pathways, it may be possible to target those interactions to treat disease.
From Germany to California
Hoffmann grew up in the Lake of Constance region of southwest Germany, at the foot of the Alps, where his passion for science was at first obscured by his love of sports. As a child, his dream was to become a tennis player—and he chased that dream by spending most of his time and focus on the tennis court.
Yet, he credits tennis for sparking his curiosity about the human body, especially the biochemistry and physiology that leads to peak athletic performance.
When it was time for college, Hoffmann chose a sports science program at Loughborough University in England. There, he developed a keen interest in medicines and how they function in the body.
“I became fascinated with pharmacology,” he says.

Among his many scientific aims at Gladstone, Hoffmann is working to understand the biological mechanisms that influence how our immune system responds to vaccines—and then capitalize on that knowledge by creating more effective vaccine technologies.
After graduating, he once again followed his curiosity, enrolling in a pharmacy program at University of Bath, where he earned his master’s degree. During that time, he accepted a research internship at Bristol Myers Squibb, working hand-in-hand with scientists for the first time. The experience allowed Hoffmann to immerse himself in the world of scientific research, confirming it was the direction he wanted to take his career.
“I was frustrated with how limited our treatment options are for disease and I started to realize that I might be able to make a bigger difference in this world if I could develop new therapies,” he says.
Hoffmann made another important realization at that time: He needed a change of scenery. After eight years in the notoriously damp, gray climate of England, he sought out doctoral programs in parts of the world with plentiful vitamin D. He landed at Caltech—a top biology program that also happens to be located in Pasadena, California, which gets about 300 days of sunshine per year.
Translating Discoveries to the Patient
Hoffmann’s vaccine work at Caltech garnered him a prestigious NIH Director’s Early Independence Award in 2022. This allowed him to skip traditional postdoctoral training and move immediately into an independent research position at Caltech. He opened his own lab, serving as the inaugural Merkin Institute Fellow at Caltech’s Merkin Institute for Translational Research.
As Hoffmann now ramps up his lab at Gladstone, he remains focused on his goal to impact patients’ lives.
“Gladstone’s collaborations with UC San Francisco and industry partners provide a unique opportunity to translate research into biotechnologies and therapies that can help patients,” Hoffmann says. “What more could a scientist ask for?”
Featured Experts
Gladstone’s Scientific Highlights of 2025
Gladstone’s Scientific Highlights of 2025
From fundamental insights to translational advances, here’s how Gladstone researchers moved science forward in 2025.
Gladstone Experts Alzheimer’s Disease Autoimmune Diseases COVID-19 Neurological Disease Genomic Immunology Cardiovascular Disease Data Science and Biotechnology Infectious Disease Conklin LabBeyond Viruses: Expanding the Fight Against Infectious Diseases
Beyond Viruses: Expanding the Fight Against Infectious Diseases
The newly renamed Gladstone Infectious Disease Institute broadens its mission to address global health threats ranging from antibiotic resistance to infections that cause chronic diseases.
Institutional News News Release Cancer COVID-19 Hepatitis C HIV/AIDS Zika Virus Infectious DiseaseHow Do Vaccines Work?
How Do Vaccines Work?
An in-depth explainer about different types of vaccines and what happens in the body after vaccination.
Gladstone Experts COVID-19 Deep Dive