Gladstone NOW: The Campaign Join Us on the Journey✕

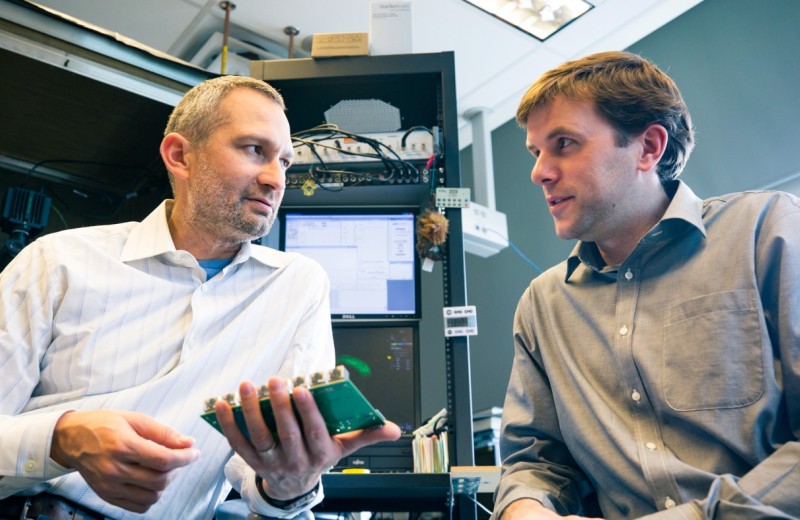
Nevan Krogan, PhD, and Anatol Kreitzer, PhD, hope to develop more focused treatments that target specific proteins or neurons implicated in many psychiatric disorders. [Photo: Chris Goodfellow, Gladstone Institutes]]
For most psychiatric disorders, treatment options are limited. Virtually no new drug targets have emerged since the psychiatric boom of the 1950s, which led to the antidepressants, antipsychotics, and antianxiety drugs that are still prescribed today. What’s more, the drugs that do exist are far from perfect, often treating the symptoms rather than the root causes of disease.
This problem is especially true for conditions that fall outside of the three major categories of psychiatric drugs. For example, autism, obsessive-compulsive disorder (OCD), and Tourette syndrome are all treated with antidepressant or antipsychotic drugs, even though their underlying biology and symptoms are very different.
Two scientists at the Gladstone Institutes are each working in areas that may improve the treatment of psychiatric disorders. By studying the basic connections and functions of brain cells, they hope to identify new drug targets for conditions, such as autism and OCD.
Mapping the Roots of Autism
Gladstone Senior Investigator Nevan Krogan, PhD, is studying how the proteins in neurons interact with each other and how mutations in the proteins lead to autism.
“In neuroscience, there is a huge black box between genetic information and behavior,” said Krogan, who is also the director of the Quantitative Biosciences Institute and a professor of cellular and molecular pharmacology at the University of California, San Francisco (UCSF). “We want to understand how genes influence behavior and what’s happening at a mechanistic level in the neurons. We think that proteins are the key to understanding these mechanisms.”
Genome mapping initiatives have been tremendously successful, producing a tsunami of genetic information about diseases ranging from cancer to schizophrenia. But translating this information into treatments has proved to be much more difficult.
“The initial idea was to determine the DNA sequence of patients with autism, for instance, find the one or two mutated genes that cause the disease, and develop drugs to overcome the mutations’ negative effects,” explained Krogan. “In reality, there are a vast number of genes involved in any given disease, and several different mutations may exist in the same gene.”
Krogan is going to the next level and studying the proteins that are controlled by the mutated genes. Working in collaboration with autism genetics experts Matthew State, MD, PhD, chair of the UCSF Department of Psychiatry, and Jeremy Willsey, PhD, an assistant professor at the UCSF Institute for Neurodegenerative Diseases, Krogan’s laboratory is studying 65 genes that have been implicated in autism. Their objective is to map the interactions among the 65 proteins those genes code for and chart the networks between proteins, like drawing constellations out of stars in the sky. To help accomplish this goal, Krogan, State, and Willsey have formed the Psychiatric Cell Map Initiative (PCMI), which is focused on using network biology to help understand autism and other psychiatric disorders.
Through their connections, the initial 65 proteins could easily sprout to a web of 600 proteins. So the scientists’ next step will be to go back to the genes and organize them into pathways. Using CRISPR genome editing in human neurons, Krogan’s team will delete all 600 genes, one by one as well as in combination, to determine how each one affects the neurons’ health. They expect that many genes will overlap and affect the same cellular process, which will help them narrow down the number of potential drug targets.
“It might initially appear that the 65 genes identified in genome mapping are disconnected, but if you look at the bigger picture of the proteins they influence, you may see that the genes are involved in the same system and can all be targeted with the same treatment,” said Krogan. “Our goal is to find key nodes that are responsible for the underlying biology behind autism and that could be ultimately targeted to treat the disorder.”
Tracking What Happens When the Brain Gets Stuck
Also contributing to the understanding of brain function, Gladstone Senior Investigator Anatol Kreitzer, PhD, studies the connections neurons make with each other in the brain.
Kreitzer is applying knowledge of an area in the brain called the basal ganglia—gained through research on Parkinson’s disease—to pursue answers about decision-making and motivated behaviors. The basal ganglia receives substantial input from neurons that make dopamine, a neurochemical that is essential for fundamental functions, such as movement, learning, motivation, and reward.
“The circuits in the brain that control movement also control motivation,” explained Kreitzer, who is the director of the neuroscience graduate program at UCSF. “These pathways evolved to direct us toward more favorable options and to deter us from less favorable options.”
One feature of many psychiatric diseases, including OCD, addiction, autism, and schizophrenia, is a lack of cognitive flexibility. Most people can adapt their behaviors to their environment, but some people get stuck performing certain actions even when they are no longer beneficial or necessary. For example, a person with OCD may wash their hands dozens of times, even though they know they are clean.
This type of fixed, inflexible behavior is called perseveration, and Kreitzer thinks it is due to an impairment in the basal ganglia.
“Our goal is to understand the underlying mechanisms of value-based decision-making—how cells in the basal ganglia contribute to this ability to respond flexibly,” he said. “It is impossible to understand disease if you don’t understand the basic framework of how that system works. Once we define how this system works, we can leverage that information to treat conditions that are characterized by cognitive inflexibility or perseveration.”
For the scientists in Kreitzer’s laboratory, the first step is to identify how neurons in the basal ganglia fire when a decision is made. They hope to distinguish between the cells that are sensitive to choice and reward (allowing a mouse to flexibly adjust its behavior to obtain a reward) and the cells that become insensitive to reward (causing a mouse to become stuck repeating the same behavior, regardless of outcome).
After they identify these pathways, the researchers can start to manipulate the neurons to change the behavior. Eventually, Kreitzer hopes to develop therapies that target only the neurons that do not adapt appropriately.
“If we can identify a cell type that’s engaged during this behavior, then we have a new drug target,” he said. “This sort of treatment could one day help people with OCD or other conditions characterized by perseveration.”
Moving the Needle Towards Better Treatments
Both Krogan and Kreitzer hope to develop more focused treatments that target the specific proteins or neurons implicated in disease. This approach could reveal more effective therapies and also identify drug targets that would result in fewer side effects. What’s more, the methods they are using can be applied to numerous disorders and mental processes, so the benefits of their work could extend well beyond autism or OCD.
“This work is in its infancy, but it’s what I’m most excited about in terms of moving the needle in a short period of time,” Krogan said. “There is so much that we still don’t know about psychiatric disorders, so while there is a lot of work that needs to be done, there is enormous potential for what we can accomplish. I think we’re going to have some big discoveries in the next few years.”
Featured Experts
Support Discovery Science
Your gift to Gladstone will allow our researchers to pursue high-quality science, focus on disease, and train the next generation of scientific thought leaders.
Young Scientists Committed to Finding Treatments for Neurodegenerative Diseases
Young Scientists Committed to Finding Treatments for Neurodegenerative Diseases
The 2020 Berkelhammer Scholars break new ground in research on dementias, Parkinson’s disease, and neuropsychiatric disorders
Donor Stories Parkinson’s Disease Alzheimer’s Disease Dementia Neurological Disease Akassoglou Lab Kreitzer Lab Mahley Lab Mucke Lab Nakamura LabNew Study Explains How Your Brain Helps You Learn New Skills
New Study Explains How Your Brain Helps You Learn New Skills
Gladstone scientists show how a type of neuron improves procedural learning
News Release Research (Publication) Neurological Disease Kreitzer LabProfile: Scott Owen, PhD
Profile: Scott Owen, PhD
Scott explains how his scientist parents worried he was too interested in science as a child. But that didn’t stop him from declaring he wanted to be a scientist as early as preschool.
Kreitzer Lab