Gladstone NOW: The Campaign Join Us on the Journey✕
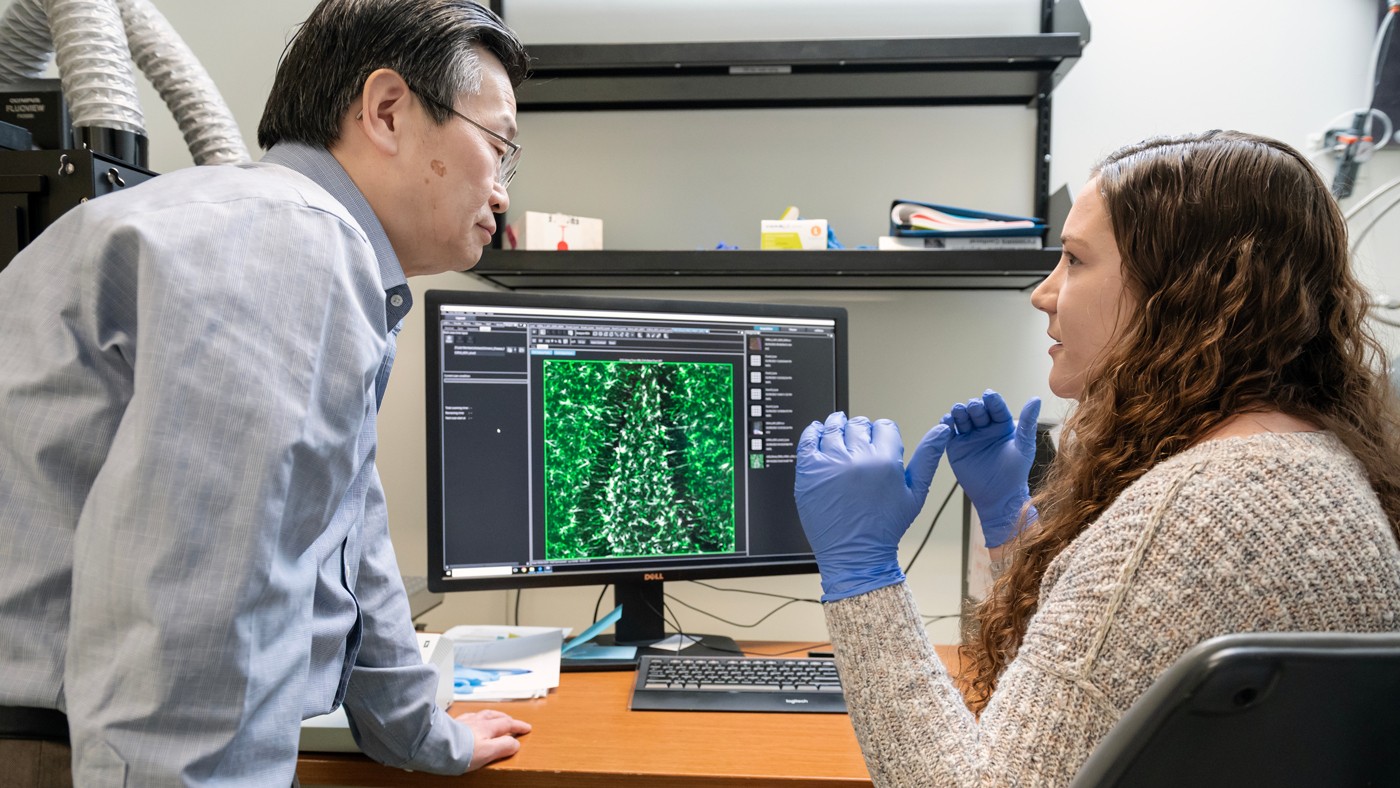
Gladstone researchers—including Yadong Huang (left) and Nicole Koutsodendris (right)—discovered how the ApoE4 gene variant makes neurons release an inflammatory signaling molecule—and how blocking that release could prevent or treat Alzheimer’s disease
If you’re one of the nearly 25 percent of people with the gene variant known as APOE4, you have a higher-than-average chance of developing Alzheimer’s disease. But while scientists have long known that APOE4 leads to changes in the brain that can contribute to dementia, the exact mechanism of that effect has been unclear.
Now, scientists at Gladstone Institutes have discovered that APOE4-producing neurons release an immune signaling molecule called HMGB1 at much higher rates than neurons producing other APOE variants. Upon release, HMGB1 activates brain immune cells called microglia, which then trigger inflammation and the degeneration of neurons.
As described in their study recently published in Cell Reports, when the researchers blocked the release of HMGB1 with a mixture of two experimental drugs, mouse models producing APOE4 and other dementia-causing factors showed much less microglial activation and neurodegeneration in the brain.
“We were quite surprised and excited that targeting this pathway leads to such strong protection against APOE4-driven neurodegeneration,” says Gladstone Investigator Yadong Huang, MD, PhD, who is the lead author of the new study and also a professor of neurology and pathology at the University of California, San Francisco. “It helps answer longstanding questions in the field about the role of APOE4-induced neuroinflammation in Alzheimer’s and also points toward new ways to treat the disease.”
Which Comes First: Neurodegeneration or Inflammation?
In the brain, Alzheimer’s disease has diverse effects on different cell types. Among the changes associated with disease are the accumulation of tau and amyloid proteins, the activation of microglia to promote inflammation, and the degeneration and ultimate death of neurons. But scientists have been unsure which of these triggers the others.

Gladstone Investigator Yadong Huang (left) is senior author of the new study published in Cell Reports.
“It could be that neurons degenerate first, and that triggers the activation of microglia,” says Huang. “But it could also be that microglia become activated first and then trigger the neurodegeneration.”
To answer this chicken-or-egg question, Huang’s team investigated microglial activation in the context of APOE4, which they have long studied as a major contributor to the genetic risk of Alzheimer’s disease.
APOE4 is one of three versions of the APOE gene. People with one copy of the APOE4 version (almost a quarter of many populations) are 3.5 times more likely to develop Alzheimer’s than those with the more common version, APOE3. People with two copies of APOE4 (about 3 percent of the population) have a 12-fold increased risk.
Huang and colleagues, including Nicole Koutsodendris, PhD, a former graduate student in Huang’s lab and lead author of the new study, asked whether neurons with the APOE4 variant produced any signaling molecules capable of activating microglia. They quickly homed in on the immune molecule HMGB1, which is known to trigger strong inflammatory responses in some cancers and infections.
In early experiments, the scientists looked to see where the HMGB1 molecule was located in neurons in the brains of Alzheimer mouse models carrying the APOE3 or APOE4 variants.
“We saw really striking patterns,” says Koutsodendris. “When neurons with APOE4 were exposed to stress, HMGB1 moved from the nucleus to the cytoplasm of the cells and was then released into the space outside the cell, whereas in neurons with APOE3, HMGB1 remained inside the nucleus.”
Additional studies in mouse models showed that selectively removing APOE4 from neurons stopped HMGB1 from being released from neurons, solidifying the link between neuronal APOE4 and the release of HMGB1.
Pointing Out a New Drug Target
Finally, the scientists studied the impact of two experimental drugs that block HMGB1 release. They showed that a mixture of both drugs reduced the activation of microglia and the development of neurodegeneration in dementia-related mouse models producing APOE4.

The team behind the study believe that drugs targeting HMGB1 could eventually be used to prevent or treat APOE4-related Alzheimer’s disease.
“It was a very clear result: when you block the exit of HMGB1 out of neurons, you protect these mice from many different aspects of Alzheimer’s disease pathology,” says Huang.
APOE4 also has other impacts on neurons, but, based on their findings, the researchers hypothesize that without the activation of microglia, these other effects aren’t sufficient to contribute to neurodegeneration. Therefore, Huang and his team believe that drugs targeting HMGB1 could eventually be used to prevent or treat APOE4-related Alzheimer’s disease.
“These small molecules have already been in clinical trials for other diseases, and so they could potentially move quickly into trials for Alzheimer’s disease,” says Huang. “But we’re also continuing our research and exploring other ways, including new drugs, that could target HMGB1, its release from neurons, and its impact on microglia.”
For Media
About the Study
The paper, “APOE4-promoted gliosis and degeneration in tauopathy are ameliorated by pharmacological inhibition of HMGB1 release,” was published in the journal Cell Reports on October 19, 2023. Other authors are: Jessica Blumenfeld, Ayushi Agrawal, Michela Traglia, Oscar Yip, Antara Rao, Min Joo Kim, Maxine R. Nelson, Yung-Hua Wang, Brian Grone, Yanxia Hao, Reuben Thomas, Misha Zilberter, Seo Yeon Yoon, and Patrick Arriola of Gladstone.
The work was supported by the National Institutes of Health (R01AG071697, R01AG076647, P01AG073082, F31AG074672, and F31AG074690).
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Featured Experts
Support Discovery Science
Your gift to Gladstone will allow our researchers to pursue high-quality science, focus on disease, and train the next generation of scientific thought leaders.
New Tool Reveals the Secrets of HIV-Infected Cells
New Tool Reveals the Secrets of HIV-Infected Cells
Developed by Gladstone scientists, HIV-seq could uncover new opportunities for treating HIV.
News Release Research (Publication) HIV/AIDS Infectious Disease Roan LabVitamin B3 Therapy Offers Hope for Fatal Childhood Disease
Vitamin B3 Therapy Offers Hope for Fatal Childhood Disease
A new framework that matches vitamins with genetic diseases helped uncover that high-dose vitamin B3 can dramatically extend survival in mice with NAXD deficiency.
News Release Research (Publication) Rare Diseases Jain LabGladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Gladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Roan has made great strides in understanding how persistent viruses including HIV cause disease and how immunity to viruses shapes human health.
Awards News Release COVID-19 HIV/AIDS Infectious Disease Roan Lab




