Gladstone NOW: The Campaign Join Us on the Journey✕

Benoit Bruneau answers questions about how his team filmed a three-dimensional movie of a heart as it develops inside a living mouse embryo.
For decades, scientists have studied mammalian heart development by taking static snapshots of mouse hearts at different stages of embryonic development. Together, the images tell a kind of stop-motion story of how cells divide, multiply, and form structures over the course of hours and days.
Now, researchers at Gladstone Institutes have used cutting-edge microscopy and genetic methods to film a real-time, three-dimensional movie of a heart as it develops inside a living mouse embryo. The approach lets them track the paths of individual cells as the heart develops, and revealed unexpected patterns in cell movement that weren’t captured in previous static images.

Martin Dominguez, first author of the study, helped gain new insight into how a healthy heart develops and how the process changes with a genetic mutation that causes heart defects. Photo courtesy of Martin Dominguez.
The new work was published in the journal Cell and led by Benoit Bruneau, PhD, a Gladstone senior investigator and director of the Gladstone Institute of Cardiovascular Disease, and Martin Dominguez, MD, PhD, a former postdoctoral scholar in his lab. Two other lab members were also involved in the study: former graduate student Alexis Leigh Krup, PhD, and postdoctoral fellow Jon Muncie, PhD.
We sat down with Bruneau to hear more about the research.
Why Does Your Lab Want to Understand Heart Development at This Level of Detail?
Congenital heart defects are the most common birth defects. We need to understand the initial genesis of the heart if we want to discover new ways of preventing or treating these defects. Once you know the fine details of how a healthy heart develops, you can start to get insight into how that process changes when a genetic mutation causes a heart defect.
We already know many of the gene mutations associated with congenital heart disease, and we also know the signaling molecules that are critical for heart development. But getting an understanding of how those genes and molecules impact cellular behavior at the whole organ level has been more challenging.
Why Has It Been So Hard to Visualize How the Heart Develops?
You can grow human or mouse heart cells in a dish, but they won’t form a three-dimensional, beating heart. That only happens inside an embryo, which makes it extremely hard to take images and impossible to get any kind of video.
Okay, So How Did You Overcome This Challenge?
A few years ago, a group of scientists grew mouse embryos in a dish under a light sheet microscope, which shines laser beams through the embryo to illuminate it. This team was able to assemble a three-dimensional video and track single cells as the embryo developed. It was a technological tour de force, and I immediately wanted to apply this approach to study the heart.

Bruneau (left) and Muncie (right) combined new technology with computational methods to capture a video of individual cells inside a developing heart.
In my lab, we have a unique line of mice that are genetically engineered so that cardiac precursor cells—which give rise to the heart—give off fluorescence. Pairing the embryo microscopy approach with our fluorescent mice enabled us to visualize each heart cell. We had to do a lot of new programming to take images of the heart from multiple angles and keep the microscope in focus, and we used new computational methods to sharpen the images and track each cell individually.
What Was It like to See These Moving, Three-Dimensional Videos for the First Time?
It was really the most amazing thing I’ve ever seen. I immediately thought “Oh my gosh, this is nuts!”
You press play on these videos and you get to watch the cells dancing all around. At first, they’re far more chaotic than we would have ever guessed. We saw many things in the videos that I never would have imagined were going on—even after 25 years of looking at static images. It really blew my mind.
What Exactly Did the New Videos Teach You?
Being able to see the movement of each cell was critical to gain a new understanding of how their behavior leads to the emergence of the heart.
When you track individual cells, you find that initially they’re moving all over the place and crossing paths, and then over time they start to march in synchrony. In one step of development, there is a thick layer of unmoving cells at the bottom of the developing organ but looser, mobile cells above; we never would have been able to tell the difference between these cells from static photos. During another step of heart development, we were able to watch how the developing embryo actually pulls heart precursor cells forward from below. It gives us a completely new concept of how the heart tube is formed.
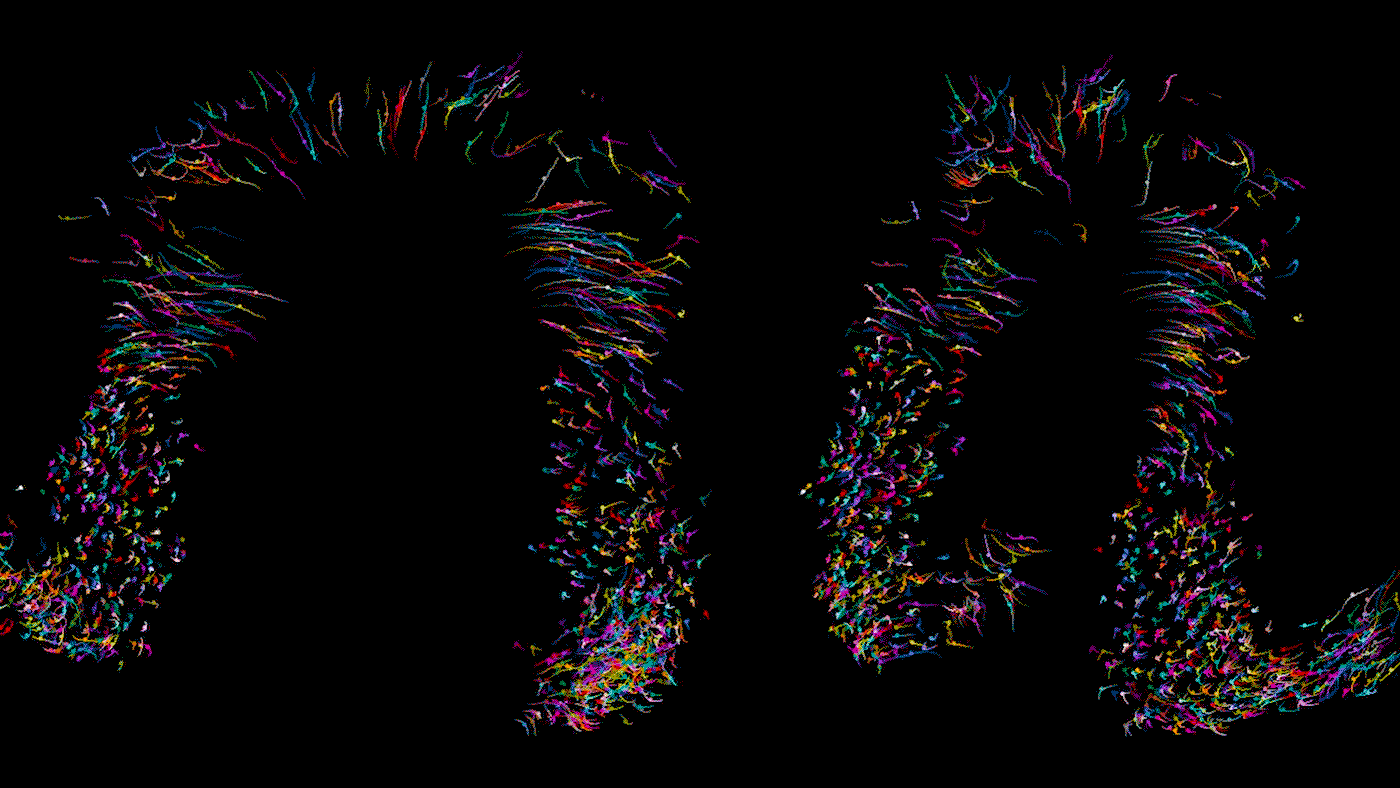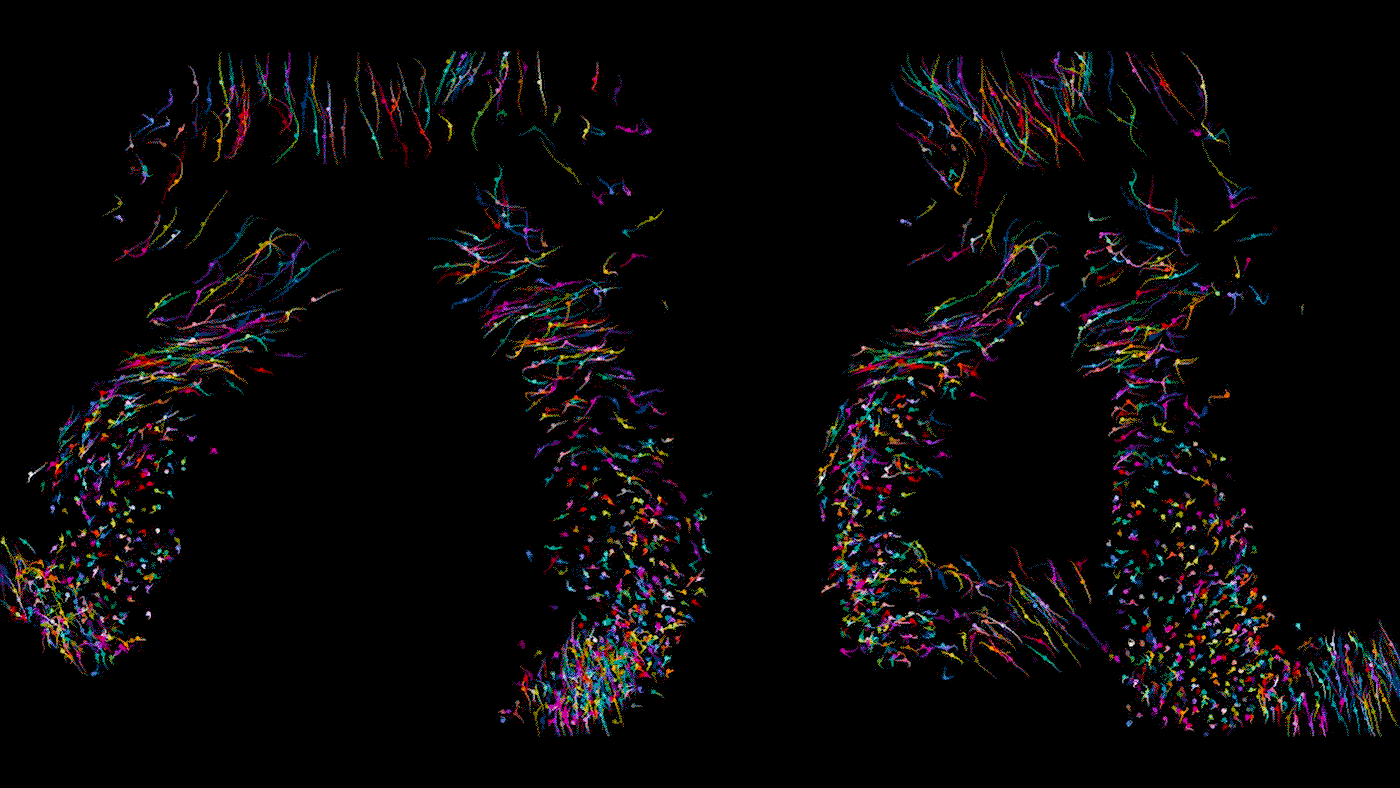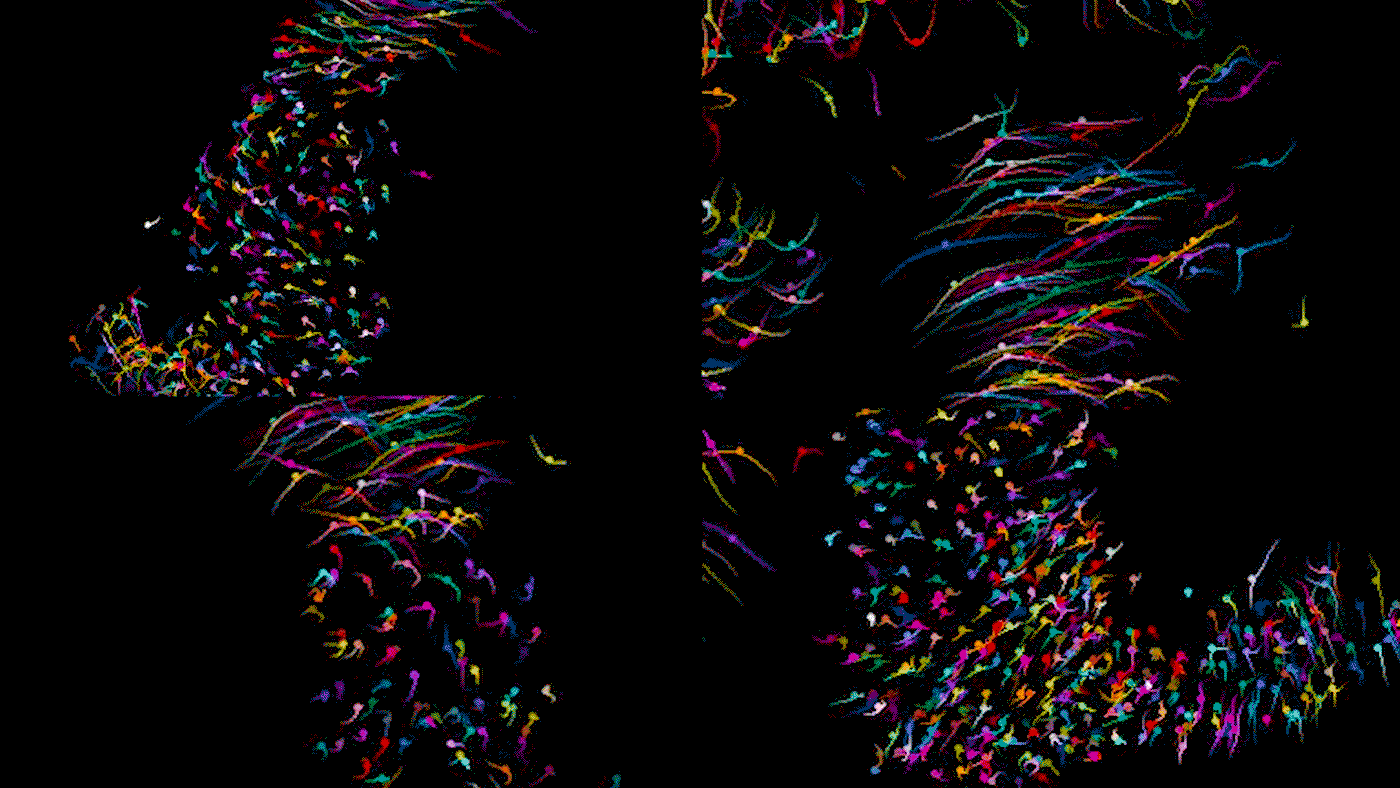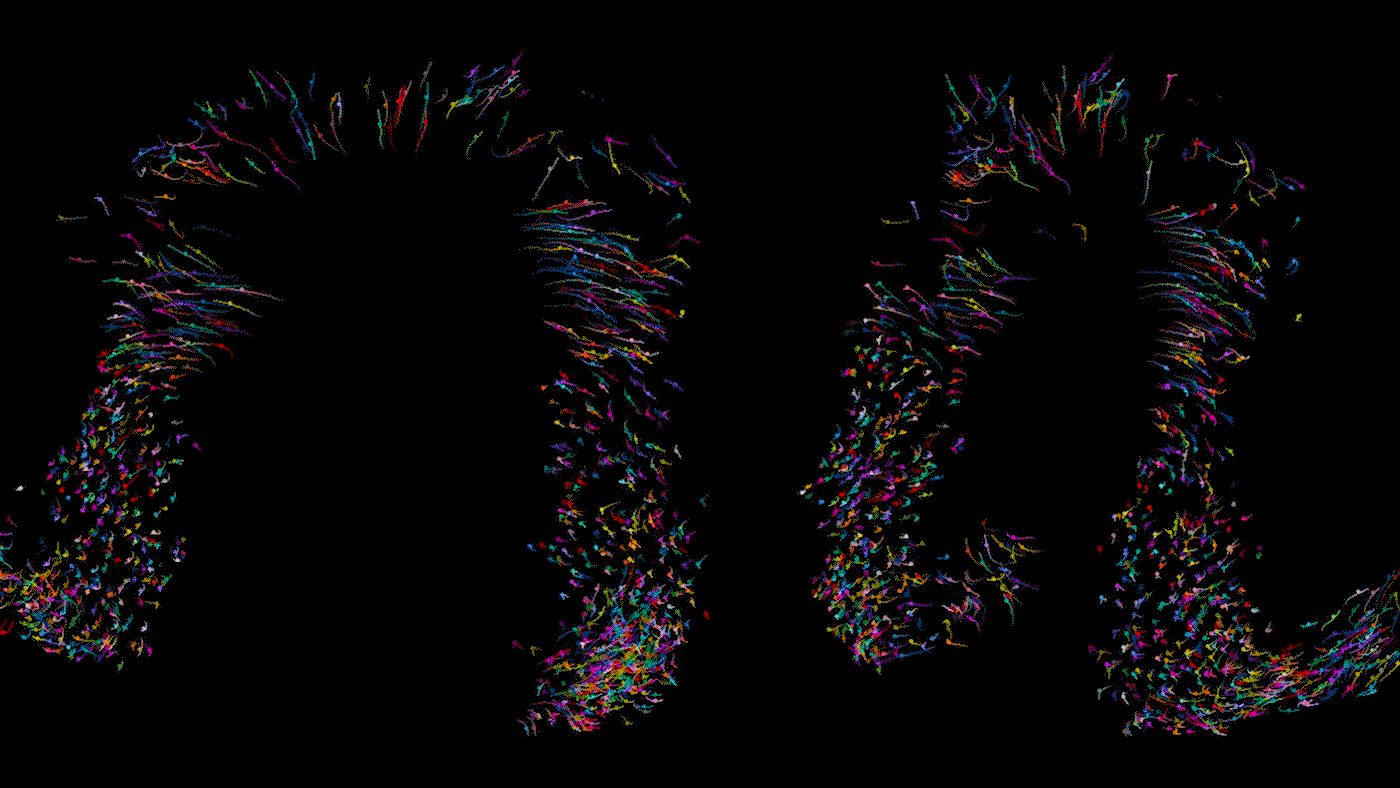
Time lapse video of a developing heart in a living mouse. Each dot is a cell in the heart, moving across 9 hours of development to form some of the important structures of the organ. As Bruneau describes, the cells shown here are in the stage where they are moving in synchrony.
In this study, we examined dozens of healthy embryos as well as embryos with a mutation in Mesp1, a gene associated with congenital heart defects. We found that in the mutant embryos, the cardiac precursor cells don’t move in the right direction; they’re stuck turning circles in random directions.
Do You Think This Approach Will Be Useful for Researchers Studying the Development of Other Organs?
Absolutely. I think people will be using this kind of live embryo microscopy coupled with fluorescent reporters for all kinds of developmental questions in the future. It’s incredibly exciting to be able to see these processes as they happen. Importantly, the microscope we used is a commercially available device, unlike the complex custom-built ones the other group used, and the software we developed is freely available and relatively easy to use. This helps democratize this sort of approach.
The paper “Graded mesoderm assembly governs cell fate and morphogenesis of the early mammalian heart” was published in the journal Cell on February 2, 2023.
The work was supported by the National Institutes of Health (R01 HL114948, 2T32-HL007731-26, T32-HL007843-24, 5T32-HL007544-34, 1F32-HL162450-01, C06 RR018928), the Younger Family Fund, the Division of Cardiology in the Department of Medicine at UC San Francisco, the National Science Foundation (GRFP 2034836), as well as the American Heart Association and the Children’s Heart Foundation (817268).
Support Discovery Science
Your gift to Gladstone will allow our researchers to pursue high-quality science, focus on disease, and train the next generation of scientific thought leaders.
New Tool Reveals the Secrets of HIV-Infected Cells
New Tool Reveals the Secrets of HIV-Infected Cells
Developed by Gladstone scientists, HIV-seq could uncover new opportunities for treating HIV.
News Release Research (Publication) HIV/AIDS Infectious Disease Roan LabVitamin B3 Therapy Offers Hope for Fatal Childhood Disease
Vitamin B3 Therapy Offers Hope for Fatal Childhood Disease
A new framework that matches vitamins with genetic diseases helped uncover that high-dose vitamin B3 can dramatically extend survival in mice with NAXD deficiency.
News Release Research (Publication) Rare Diseases Jain LabRed Blood Cells Soak Up Sugar at High Altitude, Protecting Against Diabetes
Red Blood Cells Soak Up Sugar at High Altitude, Protecting Against Diabetes
New study shows red blood cells act as hidden glucose sponges in low-oxygen conditions, explaining why people living at high altitude have lower diabetes rates and pointing toward new treatments.
News Release Research (Publication) Diabetes Jain Lab




