Gladstone NOW: The Campaign Join Us on the Journey✕
Barbara Engelhardt and her colleagues develop a new tool to align data from tissue slices virtually, expanding possibilities for 3D analysis.
Imagine a few roughly cut slices of bread on a plate. With just those slices, could you picture, in fine detail, the loaf they came from?
Now, imagine several thin slices of tissue from, say, a small tumor. You’ve tested which of several genes are active at every point across each slice’s length and width. With that two-dimensional data from just a few slices, could you predict which of the genes are active throughout the entire three-dimensional structure of the tumor? Not easy, right?
Discerning the 3D makeup of a tumor—or other tissue—using data from just a few slices is a serious computational challenge. But a new method developed at Gladstone Institutes enables researchers to do just that. This approach, published in the journal Nature Methods, could allow for much deeper understanding of biological tissue samples.
Engelhardt (left), seen here with postdoc Archit Verma (right), developed a method to visualize data from two-dimensional tissue slices in three-dimensional space.
“Without that third dimension, you can miss a lot of what’s happening in tissue,” says Gladstone Senior Investigator Barbara Engelhardt, PhD, senior author of the study. “Putting together slices in 3D space should help us begin to answer questions for which 2D data falls short. For instance, what are the precise boundaries of a tumor? Where have immune cells infiltrated the tumor? Where in the tumor would be best to inject a treatment?”
The new method, named Gaussian Process Spatial Alignment (GPSA), is not just for tumors. It can be applied to nearly any kind of tissue and any type of data obtained from tissue slices, such as the structure of cells or which genes or proteins are switched on within them—with broad implications for research and medicine.
Filling in the Blanks
One of the most widely used ways to understand biological tissue—whether from a patient with an illness or an animal in a lab—is to surgically remove some of the affected tissue and analyze it. In labs around the world, technicians may slice the removed tissue into thin pieces to view under a microscope or to test for the presence of specific molecules that could aid diagnosis, guide treatment, or hint at how well a drug is working.
However, the time, budget, and computational power needed to analyze each slice means that researchers and doctors are often limited to just a few slices from different parts of the tissue. What’s more, tissue slices become physically warped when they are cut, processed, and analyzed in a lab, making it difficult to discern exactly how the slices line up and fit together within the overall 3D structure of the original tissue.
“The first step in going from 2D slice data to a full, 3D picture of the tissue is to computationally reverse warping so that we can realign the slices in virtual space,” says Engelhardt, who is also a professor in the Department of Biomedical Data Science at Stanford University.
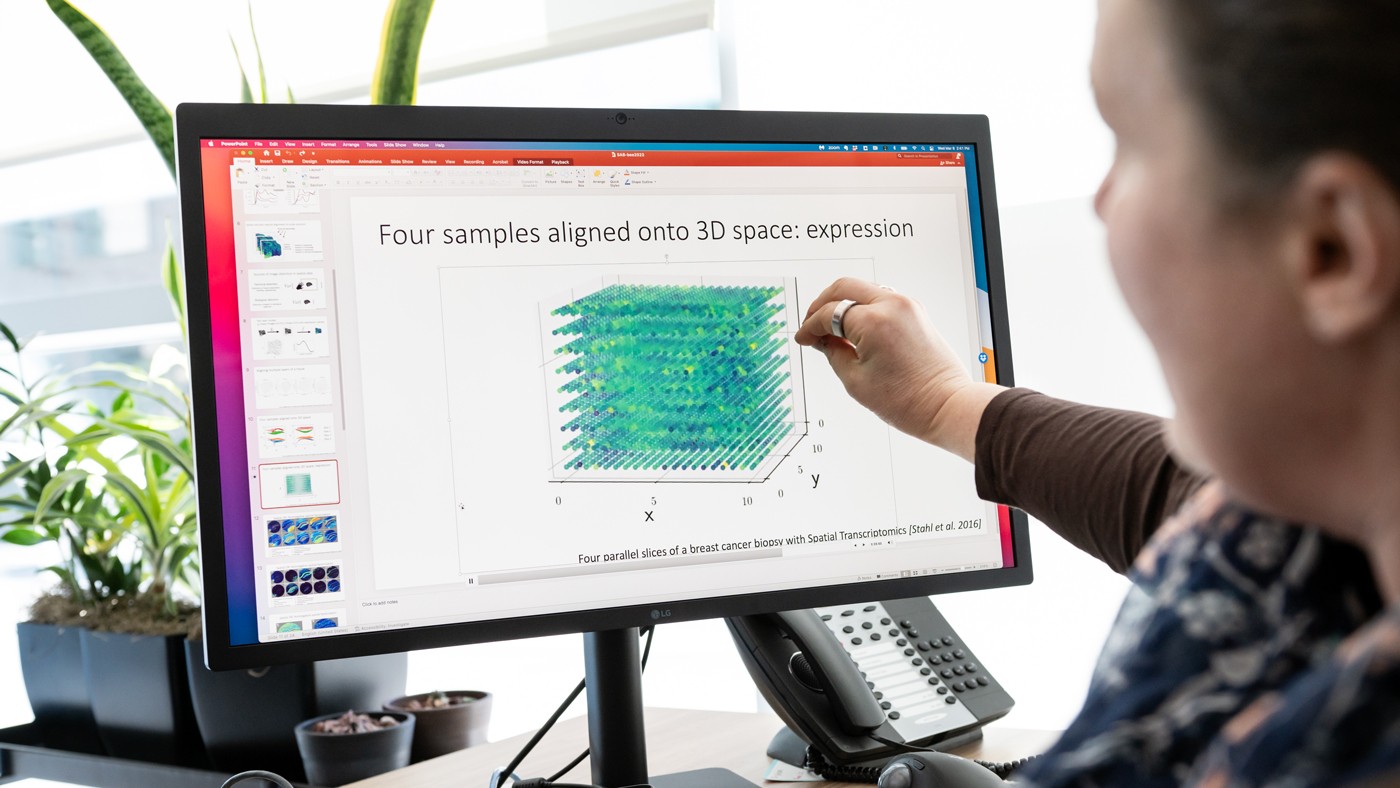
![Four parallel slices of a breast cancer biopsy with Spatial Transcriptomics [Stahl et al. 2016]](https://hello.gladstone.org/sites/default/files/inline-images/media1_AdobeExpress%281%29.gif)
The new GPSA method can generate a 3D atlas of tissue slices. In this example, four parallel slices of a breast cancer biopsy are aligned in 3D space.
To address this challenge, the GPSA method uses what Engelhardt and her team refer to as a two-layer Gaussian process. This statistical approach harnesses data from the 2D tissue slices and, in the first layer, fits the warped 2D slice onto a 3D model of the tissue. In the second layer, GPSA attributes to each point in the 3D model some data collected from the slice, such as what genes are turned on at that point. In this way, GPSA reverses warping virtually and enables a highly precise alignment of the slices.
During this process, the GPSA model fills in the spaces between slices with predictions of gene or protein expression for every point throughout the tissue, ultimately generating a 3D “atlas” of the tissue.
“Say you have four slices from different locations in a person’s breast cancer tumor, and for every point on each slice you know which of 20,000 genes are turned on or off,” Engelhardt says. “GPSA creates a fully query-able 3D atlas where, for any single ‘x, y, z’ coordinate, for any of the 20,000 genes, we can dive in and ask: What genes are on and off at this position in the tumor? And how certain are we in this estimate?”
A Highly Flexible Framework
With GPSA, researchers can construct tissue atlases with data obtained from slices of inconsistent sizes, using different technologies, and at different scales and levels of resolution. While prior methods require the 3D scaffolds or “coordinate frameworks” to be pre-specified, GPSA estimates this 3D framework from the 2D slices alone when a coordinate framework for the tissue does not yet exist. The new method can also combine multiple types of tissue-slice data—say, both information about which genes are switched on and information about cellular structure—into a single atlas.
In addition, when applied to slices taken from the same tissue at different points in time, GPSA can generate atlases that predict how every location within the tissue changes over time. In this way, the technique could help deepen understanding of aging, how illnesses progress, or how different tissues develop in a growing organism.

Engelhardt's goal is for her model to allow other researchers or clinicians to easily and deeply query 3D tissues.
“Flexibility is one of the main strengths of our new tool,” Engelhardt says.
She and her team are now conducting analyses to further demonstrate that flexibility. For instance, they have developed a method that could be used by labs on a budget to determine the minimum number of tissue slices needed—and the precise locations where those slices should be cut—for GPSA to construct a tissue atlas with the desired information.
“The goal is to maximize the insights we can gain from tissue slices, in order to allow researchers and clinicians to deeply query 3D tissues that are well-studied or tumors that are unique to a patient, and ultimately improve healthcare,” Engelhardt says.
For Media
Julie Langelier
Associate Director, Communications
415.734.5000
Email
About the Study
The paper “Alignment of Spatial Genomics and Histology Data Using Deep Gaussian Processes” was published in the journal Nature Methods on August 17, 2023.
Other authors on the study include Didong Li from University of North Carolina, Chapel Hill, as well as Andrew Jones and F. William Townes of Carnegie Mellon University.
This study was funded by the Helmsley Charitable Trust (AWD1006624), the National Institutes of Health (5U2CCA233195 and R01 HL133218), and by the National Science Foundation (AWD1005627).
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Featured Experts
Gladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Gladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Roan has made great strides in understanding how persistent viruses including HIV cause disease and how immunity to viruses shapes human health.
Awards News Release COVID-19 HIV/AIDS Infectious Disease Roan LabRed Blood Cells Soak Up Sugar at High Altitude, Protecting Against Diabetes
Red Blood Cells Soak Up Sugar at High Altitude, Protecting Against Diabetes
New study shows red blood cells act as hidden glucose sponges in low-oxygen conditions, explaining why people living at high altitude have lower diabetes rates and pointing toward new treatments.
News Release Research (Publication) Diabetes Jain LabDisrupted Boundary Between Cell Types Linked to Common Heart Defects
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Gladstone scientists identified a cellular boundary that guides heart development and revealed how disrupting it can lead to holes in the heart’s wall.
News Release Research (Publication) Congenital Heart Disease Cardiovascular Disease Bruneau Lab




