Gladstone NOW: The Campaign Join Us on the Journey✕

Gladstone's Katerina Akassoglou says the next wave of discovery for neurological diseases will emerge from interdisciplinary research that accounts for key underlying factors involved in most of the major conditions. She, along with lab members and collaborators, authored a commentary in Cell's 50th anniversary neuroscience issue.
The question of what causes complex neurological diseases such as Alzheimer’s or multiple sclerosis continues to confound scientists and doctors, with the unknowns standing in the way of early diagnoses and effective treatments.
Even among identical twins who share the same genetic risk factors, one may develop a particular neurological disease while the other does not.
That’s because unlike diseases such as cystic fibrosis or sickle-cell anemia, which are caused by a single gene, most neurological disorders are associated with many—sometimes hundreds—of rare genetic variants. And on their own, these variants can’t predict who will develop disease, as neurological conditions are also strongly influenced by environmental factors and vascular risks such as high blood pressure, aging, heart disease, or obesity.
But there’s one often-overlooked thread that connects most neurological diseases, says Katerina Akassoglou, PhD, a senior investigator at Gladstone Institutes: They’re marked by a toxic immune reaction caused by blood that leaks into the brain through damaged blood vessels.
“Interactions between the brain, blood vessels, and the immune system are a common thread in the development and progression of many neurological diseases that have been traditionally viewed as very different conditions,” says Akassoglou, a senior investigator with the Gladstone Institute of Neurological Diseases and director of the Center for Neurovascular Brain Immunology at Gladstone and UC San Francisco. “Knowing that leaked blood is a key driver of brain inflammation, we can now approach these diseases from a different angle.”
She and her collaborators share their insights on this topic in a commentary article published in Cell's 50th anniversary "Focus on Neuroscience" issue.
Neutralizing the Culprit
Akassoglou and her lab have long investigated how blood that leaks into the brain triggers neurologic diseases, essentially by hijacking the brain’s immune system and setting off a cascade of harmful often-irreversible effects that result in damaged neurons.
One blood protein in particular—fibrin, normally involved in blood coagulation—is responsible for setting off this detrimental cascade. The process has been observed in conditions as diverse as Alzheimer’s, traumatic brain injury, multiple sclerosis, premature birth, and even COVID-19. However, Akassoglou and her team found that the process could be prevented or interrupted by “neutralizing” fibrin to deactivate its toxic properties—an approach that appears to protect against many neurological diseases when tested in animal models.
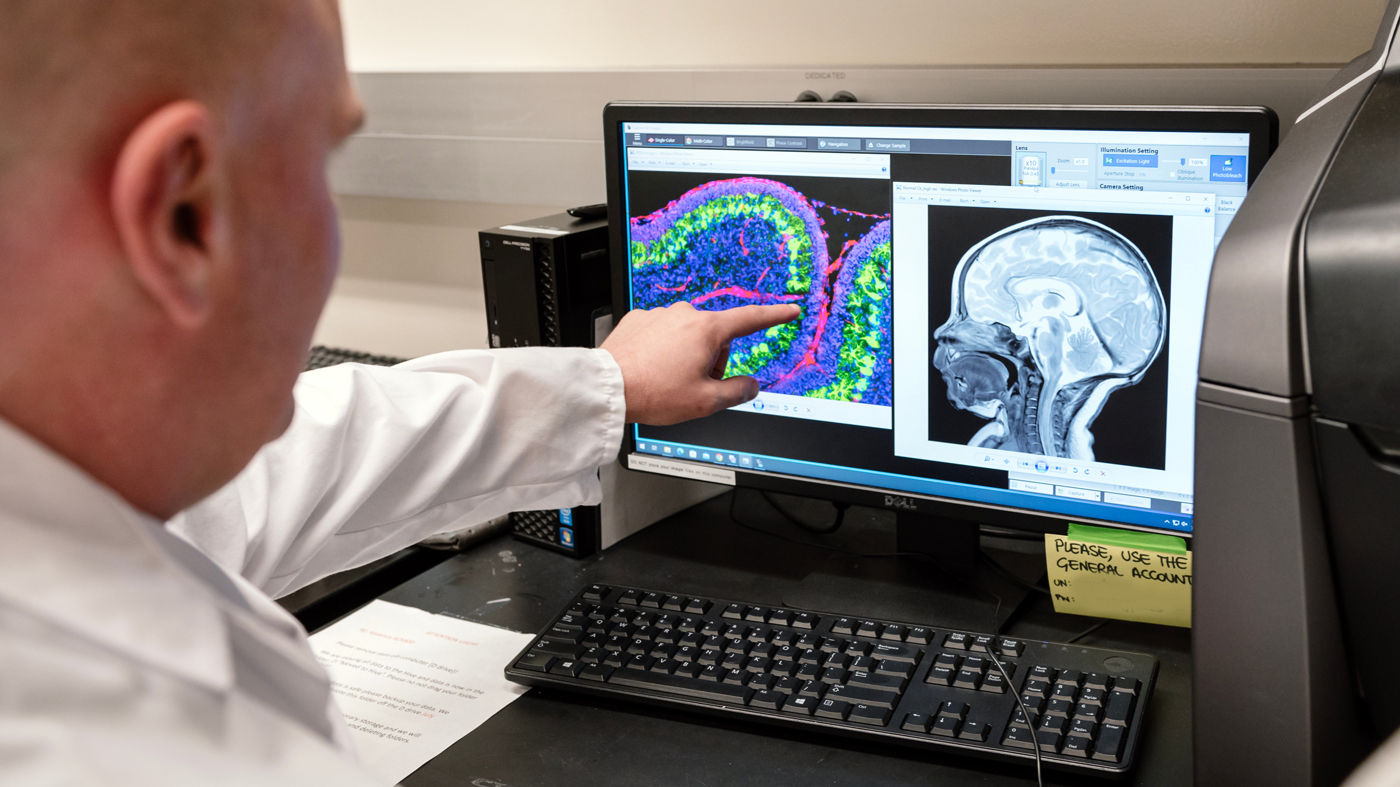
Gladstone Visiting Scientist Mark Petersen, MD, director of the Neuro-Intensive Care Nursery at UC San Francisco, worked with Akassoglou's team earlier this year to study brain bleeding in preterm infants—a common condition that's closely tied to long-term neurological issues. Here, he examines an image (on the left of his screen) of a cerebellum of a neonatal mouse with inflammatory brain injury. Petersen co-authored the new commentary article in Cell.
“As a first step, we know that neutralizing fibrin reduces the burden posed by vascular dysfunction,” Akassoglou says. Regardless of what initially caused the blood leaks, be it a head injury, autoimmunity, genetic mutations, brain amyloid or infection, neutralizing fibrin appears to be protective in multiple animal models of disease.
The scientists previously developed a drug, a therapeutic monoclonal antibody, that specifically targets fibrin’s inflammatory properties without affecting its essential role in blood coagulation. This fibrin-targeting immunotherapy has shown, in mice, to protect from multiple sclerosis and Alzheimer’s, and to treat neurological effects of COVID-19. A humanized version of this first-in-class fibrin immunotherapy is already in Phase 1 safety clinical trials by Therini Bio, a biotech company launched to advance discoveries from Akassoglou’s lab.
A New Era of Brain Research
In the Cell commentary, Akassoglou and her colleagues make the case that seemingly disparate neurological diseases must be viewed differently in light of new research on the blood-brain-immune interface.
They say that in the coming decade, scientific breakthroughs will emerge from collaborative networks of immunologists, neuroscientists, hematologists, geneticists, computer scientists, physicists, bioengineers, drug developers, and clinical researchers. These partnerships—forged across academia, industry, and foundations—will catalyze innovation in drug discovery and transform medical practice for neurological diseases.
“This is a new opportunity for drug discovery that goes beyond addressing genes alone or environmental factors alone,” Akassoglou says. “To usher in this new era, we must leverage new technologies and embrace an interdisciplinary approach that accounts for the important roles of immune and vascular systems in neurodegeneration.”

Jae Kyu Ryu, PhD (left), and Zhaoqi Yan, PhD (right), collaborate in Akassoglou's lab on a study earlier this year that revealed the blood coagulation protein fibrin is responsible for initiating toxic inflammation and neuron loss after a major head injury. Both were co-authors of the new commentary in Cell.
For Media
Kelly Quigley
Director, Science Communications and Media Relations
415.734.2690
Email
About the Study
The commentary article, “Pioneering Discovery and Therapeutics at the Brain-Vascular-Immune Interface,” appears in the October 17 issue of Cell. Authors include Katerina Akassoglou, Dimitrios Davalos, Andrew Mendiola, Mark Petersen, Jae Kyu Ryu, Christian Schachtrup, and Zhaoqi Yan.
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Featured Experts
Six Gladstone Scientists Named Among World’s Most Highly Cited Researchers
Six Gladstone Scientists Named Among World’s Most Highly Cited Researchers
The featured scientists include global leaders in gene editing, data science, and immunology.
Awards News Release Corces Lab Doudna Lab Marson Lab Pollard Lab Ye LabThe Genome Editing Playbook Is Different in Neurons
The Genome Editing Playbook Is Different in Neurons
The striking findings of a new study could influence how gene therapies are designed for many genetic diseases.
News Release Research (Publication) Neurological Disease Conklin Lab Doudna Lab CRISPR/Gene EditingEvidence Builds for Disrupted Mitochondria as Cause of Parkinson’s
Evidence Builds for Disrupted Mitochondria as Cause of Parkinson’s
A new study from Gladstone Institutes strengthens the links between energy breakdown in cells and the onset of Parkinson’s, potentially illuminating new paths for treatment.
News Release Research (Publication) Parkinson’s Disease Neurological Disease Nakamura Lab




