Gladstone NOW: The Campaign Join Us on the Journey✕

Inspired by his daughter, Nika, Dr. Benoit Bruneau researches how the heart develops in an embryo in order to find out what goes wrong in children born with congenital heart disease. [Photo: Chris Goodfellow]
My daughter, Nika, was born with a hole between the left and right chambers of her heart. Called a ventricular septal defect, it is the most common form of congenital heart disease, affecting between two and six babies out of every 1,000. While Nika’s heart was able to heal on its own, some children require surgery to close the hole, and even after the defect is corrected, the child may still experience other cardiac problems later in life.
As a cardiovascular scientist, I have dedicated my career to exploring exactly what goes wrong in hearts like Nika’s, researching the causes of congenital heart defects in my laboratory at the Gladstone Institutes. Our ultimate hope is to better treat—or even prevent—congenital heart disease.
Pinpointing the Precise Moment a Heart Starts to Form
The basis of congenital heart disease is abnormal heart development, when the heart doesn’t come together as it normally should. In order to understand this problem, we need to understand how the different parts of the heart are formed and how they come together. With that goal in mind, we designed a research project to identify the earliest signs of heart development. Our project had unprecedented results, revealing that these signs are happening much earlier than anyone believed.
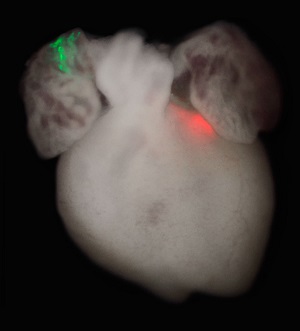 Mouse heart with two sets of labeled cell clones (red and green). Early embryo cells were genetically labeled, and the green and red colors indicate that the two clusters of cells originated from the same cells early in development. [Image: Patrick Devine]
Mouse heart with two sets of labeled cell clones (red and green). Early embryo cells were genetically labeled, and the green and red colors indicate that the two clusters of cells originated from the same cells early in development. [Image: Patrick Devine]
Using mouse embryos, we traced individual cells as they grew from the early embryo to determine the exact moment some of these cells start to signal they will become heart cells. We made two critical discoveries: the signals occur while the embryo is still just a simple cup-shaped structure, before any organs have begun to form; and, from the very beginning, these cells are earmarked for either the left or right ventricle (chamber) of the heart. What’s more, during the course of development, the cells never cross over or intermingle, fixed in their designation of left or right ventricle.
This revelation has important implications for conditions like Nika’s. While the conventional wisdom has been that congenital heart defects occur late in the formation of the organ, we now think that the defects likely result from this very first decision involving the division between the left and right ventricles. Instead of staying right next to one another like they’re supposed to, cells from the left and right ventricle might mix at some point early on, possibly causing a crack in the wall between the two chambers of the heart.
Research Results Aid the Study of New Treatments
Not only does this finding expand our knowledge of childhood cardiac disorders, it also informs our ongoing study of potential regenerative medicine treatments for adult heart disease. Complementing the work of my colleagues in the Roddenberry Center for Stem Cell Biology and Medicine at Gladstone, our efforts to learn more about how the heart forms can improve methods for creating heart cells from stem cells, either in a petri dish or inside a patient after a heart attack.
For example, the same cellular signals that guide early heart development can be used to direct stem cells to become the different heart cell types needed for modeling disease in a dish. Or these signals can help reprogram connective tissue into beating muscle cells inside the heart, effectively regenerating healthy cells from scar tissue following a heart attack.
By defining the processes behind these cellular signals, the blueprint for the heart, we hope to one day recreate them through chemical methods—using drug cocktails to coax stem cells into becoming heart cells at just the right time and place. If we understand the cues that help create all of the different types of specialized heart cells, we can leverage those cues to make better artificial heart cells. And in the not-so-distant future, we may be able to use nature’s own instructions to create an artificial heart.
Benoit Bruneau, PhD, is the Associate Director of the Gladstone Institute for Cardiovascular Disease. He is also a Professor of Pediatrics at the University of California, San Francisco.
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Disrupted Boundary Between Cell Types Linked to Common Heart Defects
Gladstone scientists identified a cellular boundary that guides heart development and revealed how disrupting it can lead to holes in the heart’s wall.
News Release Research (Publication) Congenital Heart Disease Cardiovascular Disease Bruneau LabCIRM Awards $7.5 Million in Discovery Grants to Gladstone Investigators
CIRM Awards $7.5 Million in Discovery Grants to Gladstone Investigators
Two ambitious research projects led by Gladstone investigators are boosted by funds from the California Institute for Regenerative Medicine.
Grants News Release Congenital Heart Disease Cardiovascular Disease Bruneau Lab Conklin Lab CRISPR/Gene Editing Human Genetics Regenerative MedicineMatters of the Heart: A Conversation with Gladstone’s Benoit Bruneau
Matters of the Heart: A Conversation with Gladstone’s Benoit Bruneau
Bruneau, director of the Gladstone Institute of Cardiovascular Disease, shares exciting recent advances in heart research and talks about the impact of predictive AI.
Gladstone Experts Heart Failure Cardiovascular Disease Alexanian Lab Bruneau Lab Pollard Lab Theodoris Lab AI



