Gladstone NOW: The Campaign Join Us on the Journey✕
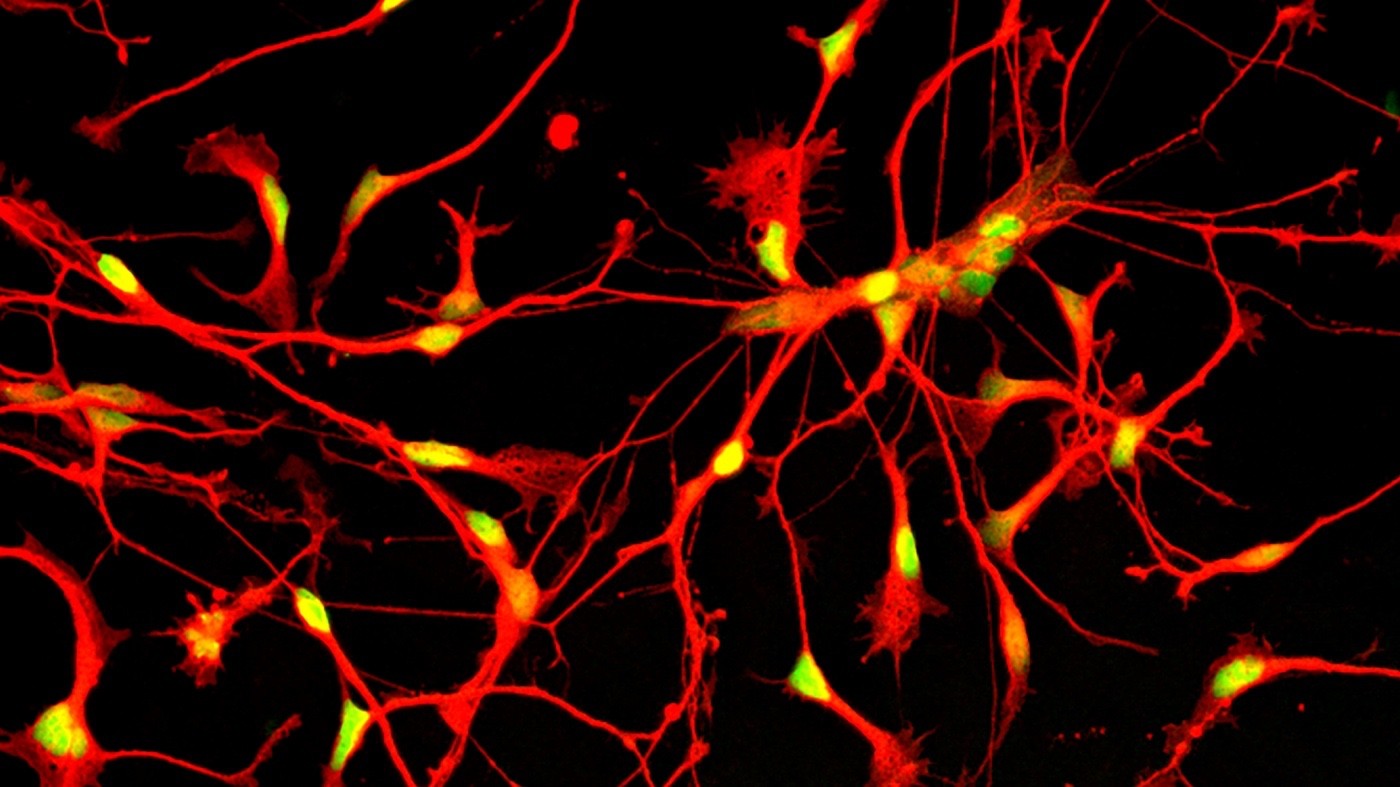
Immature human inhibitory neurons. Gladstone scientists are combining expertise and cutting-edge stem cell technology to tackle Alzheimer’s disease. [Image: Ramsey Najm, Gladstone Institutes]
Stem cells hold the promise of a cure for a host of debilitating conditions, including heart failure, diabetes, and spinal cord injuries. However, neurodegenerative disorders, such as Alzheimer’s disease, have been considered out of reach, because the underlying biology of the brain is so complicated.
“The general feeling in the field used to be that stem cell therapy for Alzheimer’s was not feasible, because the disease was too complex and too many cells would need to be replaced,” said Yadong Huang, MD, PhD, a senior investigator at the Gladstone Institutes. “Research now suggests that there might be a way to overcome this hurdle by using the right type of cell in the right place.”
Thanks to recent technological developments, new insights into brain health, and some creative thinking, two Gladstone scientists are embarking on research to replace lost or malfunctioning brain cells with new ones created from stem cells.
The Right Type of Cell
In the brain, billions of excitatory neurons act like green traffic lights, driving other cells to fire in complicated networks of activity. Inhibitory neurons serve as red lights, stopping or directing the flow of activity in the brain.
Death of inhibitory neurons may be a major feature of Alzheimer’s disease, especially in the presence of the apoE4 gene, the primary genetic risk factor for Alzheimer’s. Aging and the apoE4 protein combine to impair inhibitory neurons in the hippocampus—a key memory center that is among the first regions affected by Alzheimer’s. The loss of inhibitory neurons is linked to deficits in learning and memory, and Huang thinks that transplanting new inhibitory neurons into the hippocampus could be a viable treatment for Alzheimer’s disease.
“There are fewer inhibitory neurons than excitatory neurons in the brain, but each one has the power to stop thousands of excitatory neurons from firing,” he explained. “Replacing inhibitory neurons instead of excitatory neurons may lower the number of cells required to reverse some of the damage caused by Alzheimer’s.”
Supported by a CIRM translational grant, scientists in Huang’s laboratory are using human stem cells to create inhibitory neuron progenitors—early-stage brain cells that can develop into mature inhibitory neurons. The researchers then transplant these progenitor cells into the hippocampi of mice that carry the apoE4 gene and model aspects of Alzheimer’s disease. In preliminary studies, they found that the transplanted progenitor cells replenish the pool of inhibitory neurons and even improve learning and memory in the mice.
Treating the Brain’s Immune System
Li Gan, PhD, a senior investigator at Gladstone, is also pursuing stem cell transplants to treat Alzheimer’s disease. However, instead of focusing on neurons, she thinks an alternative strategy lies in the brain’s immune system.
Microglia—the brain’s immune cells—work to remove the clumps of amyloid protein that accumulate in Alzheimer’s disease. However, in the process, microglia turn on inflammatory pathways in the brain, which can cause further damage. Mutations in genes that control microglia were recently linked to Alzheimer’s disease in genome-wide association studies. These tests compare the DNA of thousands of people with and without a disease to identify genes linked to a higher risk for the condition.
“For decades, researchers have seen chronic inflammation in the brains of Alzheimer’s patients, but they thought it was a consequence of the disease, not a cause of it,” Gan said. “Now, thanks to genome-wide association studies, we think this neural immune response may actually contribute to Alzheimer’s disease.”
Gan is embarking on a new study to return the brain’s immune response to normal by creating microglia from stem cells and transplanting them into the brains of mouse models of Alzheimer’s disease. She plans to use CRISPR genome editing on the cells to ensure that only their protein-clearing abilities and not their pro-inflammatory properties are included. She cautions that the approach is still new and untested, but she thinks it holds promise.
“Microglia are injury response cells, so they are programmed to go to where the injury is, which suggests they may be more amenable to use as cell therapy,” Gan explained. “Also, because these cells secrete chemicals, only a small number of microglia may be needed to change the environment in the brain.”
How to Help Aging Brains
One of the challenges researchers face when developing treatments for Alzheimer’s disease is that the patients are elderly, which means their brains are less plastic and more resistant to rewiring. What is more, as Alzheimer’s disease progresses, too many neurons have died for drug therapies to be able to improve a patient’s condition.
By introducing new cells into the brain, particularly types that require only a small number to have a large effect, Huang and Gan hope to restore aged, diseased brains to a healthier state, improving the lives of those who suffer from Alzheimer’s disease.
Want to Join the Team?
Our people are our most important asset. We offer a wide array of career opportunities both in our administrative offices and in our labs.
Explore CareersGladstone’s Scientific Highlights of 2025
Gladstone’s Scientific Highlights of 2025
From fundamental insights to translational advances, here’s how Gladstone researchers moved science forward in 2025.
Gladstone Experts Alzheimer’s Disease Autoimmune Diseases COVID-19 Neurological Disease Genomic Immunology Cardiovascular Disease Data Science and Biotechnology Infectious Disease Conklin LabScience in Seconds | The Thinking Microscope: Research Powered by an AI Brain
Science in Seconds | The Thinking Microscope: Research Powered by an AI Brain
In this video, Steve Finkbeiner and Jeremy Linsley showcase Gladstone’s groundbreaking “thinking microscope”—an AI-powered system that can design, conduct, and analyze experiments autonomously to uncover new insights into diseases like Alzheimer’s, Parkinson’s, and ALS.
Gladstone Experts ALS Alzheimer’s Disease Parkinson’s Disease Neurological Disease Finkbeiner Lab AI Big DataKaterina Akassoglou Receives Zenith Fellows Award to Advance Alzheimer’s Research
Katerina Akassoglou Receives Zenith Fellows Award to Advance Alzheimer’s Research
Akassoglou has opened doors to understanding how the blood protein fibrin is involved in Alzheimer’s and other neurodegenerative diseases.
Awards News Release Alzheimer’s Disease Center for Neurovascular Brain Immunology Akassoglou Lab



