Gladstone NOW: The Campaign Join Us on the Journey✕
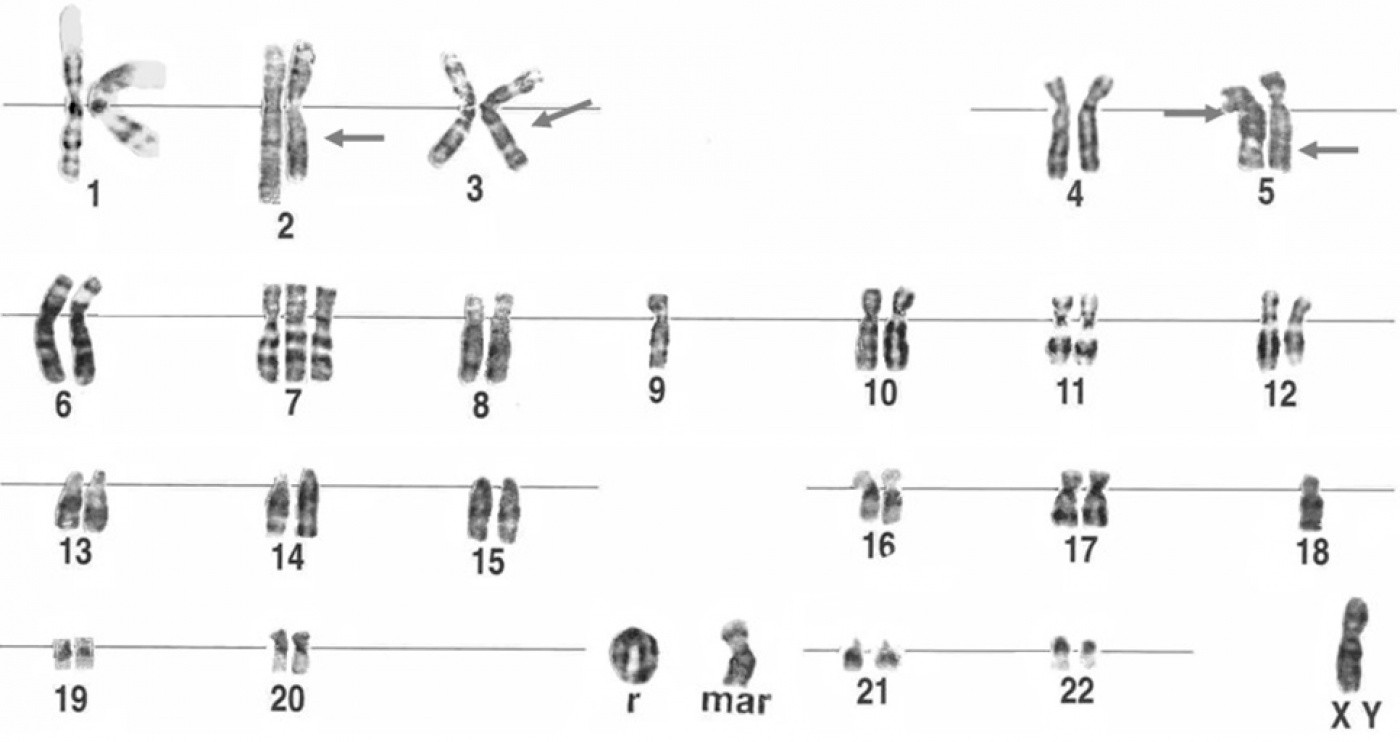
Normally chromosomes are linear. However, in rare cases, the ends of the chromosome become either damaged or broken, and the normal linear structure transforms into ring, as shown here at r. Using iPS cell technology, the research teams found a way to efficiently and accurately study how ring chromosomes form. [Figure: Cell & Chromosome]
Our DNA contains the genetic information that guides our body on how to develop and function. But sometimes, the DNA is altered—by radiation, aging or other events—and mutations and serious diseases can result. In a study published in the latest issue of the journal Nature, a team of investigators from the University of California, San Francisco (UCSF) and the Gladstone Institutes describe a new technique that will help scientists better understand how stem cells maintain proper chromosome numbers during development—and what happens when that process goes awry.
“The ability to maintain the right chromosome number and DNA integrity is absolutely critical for the development of a healthy child,” said Marina Bershteyn, PhD, a postdoctoral fellow at UCSF and the paper’s lead author. “However, how this is achieved in rapidly dividing stem cells is not completely understood.”
Before a “parent” cell divides-a process known as mitosis, two copies of each chromosome are made, and one copy is passed along to each “daughter” cell. During this process, special proteins identify DNA mutations, which then kick-start a process to either repair the damage or, if the damage is too great, kill the cell. In some cases, however, these “quality control” systems fail. They miss the mutation and the cell survives with the DNA damage intact. These types of chromosomal malformations can lead to birth defects, mental disabilities, and growth retardation.
The research team, led by former UCSF investigator Anthony Wynshaw-Boris, MD, PhD (now at Case Western Reserve University), studied a specific aberration called a ring chromosome. Normally, chromosomes are linear. However, in rare cases, the ends of the chromosome become either damaged or broken, and fuse together forming a circle, or ring. These changes in chromosome shape then affect how the cell divides and how genetic information is passed on to new cells.
The scientists took advantage of a 2007 discovery by Gladstone Investigator Shinya Yamanaka, MD, PhD. Using methods developed by Dr. Yamanaka and his research team, Dr. Bershteyn and Gladstone Postdoctoral Researcher Yohei Hayashi, PhD, the paper’s other lead author, reprogrammed adult skin cells into cells that are nearly identical to embryonic stem cells. These cells, called induced pluripotent stem cells (or iPS cells) can then develop into virtually any type of the cell in the body. This discovery, which earned Dr. Yamanaka the 2012 Nobel Prize in Physiology or Medicine, has revolutionized the fields of stem cell biology and regenerative medicine and has allowed scientists to model disease from patient-derived cells in a dish, thus working towards a new era of personalized medicine.
In this study, the team obtained skin cells from patients with Miller Dieker syndome (MDS), a brain disease characterized by a smooth brain surface and defective neuronal migration. While MDS is typically caused by deletions at one end of chromosome 17, in one of the patients the deletion was also associated with a ring chromosome. After transforming MDS skin cells into iPSCs, the team looked at the number and structure of all 23 pairs of chromosomes inside each cell.
Interestingly, the MDS iPSCs derived from skin cells with ring 17 showed little evidence of the ring chromosome. They also found that the suite of genes normally missing from chromosome 17—a hallmark of MDS—reappeared in MDS patient-derived iPSCs.
“This suggests that the reprogramming process may repair or replace the ring chromosome in these cells,” explained Dr. Hayashi. “This may have valuable implications in treating or curing diseases caused by chromosome mutations.”
How exactly does the reprogramming process fix the broken chromosome? The research team proposed two possibilities. First, another DNA break might occur in the ring chromosome sometime during reprogramming, causing the structure to open and be repaired. Alternatively, the mutated chromosome might be completely replaced with a normal chromosome.
In their analysis, the research team found that a process called compensatory uniparental disomy (UPD) was responsible for the complete replacement of the ring chromosome with a normal copy of chromosome 17. UPD occurs when a person receives two copies of a chromosome, or of part of a chromosome, from one parent and no copies from the other parent. They speculate that because the process of reprogramming increases the number of cell divisions that take place (as compared to what happens in adult skin cells), there are more opportunities for the cell cycle checkpoints to correct for the chromosomal breakage.
“Our findings are proof of principle that reprogramming can be used to repair certain chromosomal aberrations such as ring chromosomes,” said Dr. Bershteyn. “We hope that this technology will eventually evolve into chromosome therapy for various mutations, potentially improving the outlook of those suffering from a wide variety of genetic diseases.”
Heart-to-Heart Connection: Exploratorium and Gladstone Bring a Breakthrough Science Exhibit to Life
Heart-to-Heart Connection: Exploratorium and Gladstone Bring a Breakthrough Science Exhibit to Life
At San Francisco’s Exploratorium, visitors sync their heartbeat with living heart cells; the first-of-its-kind exhibit was created through the museum’s long-running partnership with scientists at Gladstone Institutes.
News Release Stem Cells/iPSCs Conklin Lab Yamanaka Lab Cardiovascular Disease Research (Publication)When Two Disruptive Technologies Converge
When Two Disruptive Technologies Converge
How gene editing and stem cells are working in tandem at Gladstone Institutes to change science and medicine
Deep Dive Stem Cells/iPSCs CRISPR/Gene Editing Srivastava Lab Yamanaka Lab Doudna Lab Conklin Lab Marson Lab Huang Lab Ott LabA Key Function for Tight Junctions in Embryo Models
A Key Function for Tight Junctions in Embryo Models
Barriers between stem cells play a crucial role in human development—and offer a new potential route for fertility treatment
News Release Research (Publication) iPS Cell Research Center Yamanaka Lab Stem Cells/iPSCs