Gladstone NOW: The Campaign Join Us on the Journey✕
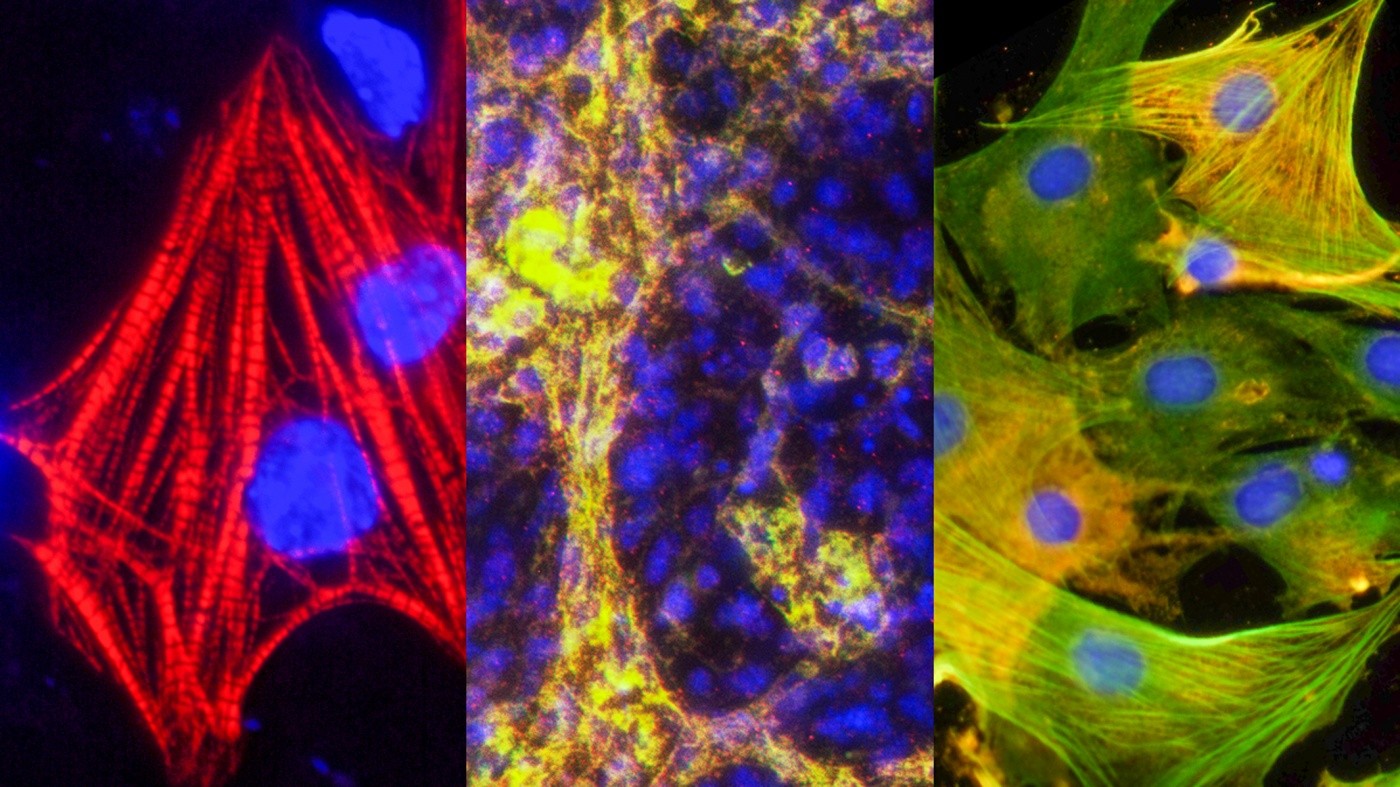
Gladstone scientists have discovered how to make the three types of heart cells—cardiomyocytes, endothelial cells, and smooth muscle cells—out of a new type of cardiac stem cell created in a lab. [Image: Yu Zhang, Gladstone Institutes]
Scientists at the Gladstone Institutes have discovered how to make a new type of cell that is in between embryonic stem cells and adult heart cells, and that may hold the key to treating heart disease. These induced expandable cardiovascular progenitor cells (ieCPCs) can organically develop into heart cells, while still being able to replicate. When injected into a mouse after a heart attack, the cells improved heart function dramatically.
“Scientists have tried for decades to treat heart failure by transplanting adult heart cells, but these cells cannot reproduce themselves, and so they do not survive in the damaged heart,” explained Yu Zhang, MD, PhD, lead author on the study and a postdoctoral scholar at the Gladstone Institutes. “Our generated ieCPCs can prolifically replicate and reliably mature into the three types of cells in the heart, which makes them a very promising potential treatment for heart failure.”
Cardiovascular progenitor cells (CPCs) are generated naturally as the heart forms in an embryo and give rise to a selection of different kinds of heart cells. In the current study, published in the journal Cell Stem Cell, the researchers were able to create CPCs in the lab. They used pharmaceutical drugs to catch and maintain heart stem cells at the cardiac precursor state before they developed into fully-functional heart cells.
Organ-specific stem cells are special because they can both develop into adult cells and replicate indefinitely. In the current study, the ieCPCs expanded exponentially over a dozen generations, creating enough cells to potentially treat numerous patients. This type of self-renewal is particularly important for treating heart failure, as more than one billion heart cells can be lost after a heart attack. Prolific cell renewal means ieCPCs could be a sustainable way to replace those damaged cells. ieCPCs can also develop into each of the three different types of heart cells: cardiomyocytes, endothelial cells, and smooth muscle cells. When injected into a heart, the cells spontaneously transitioned into these cells without needing any additional signals.
Previous attempts to treat heart failure by transplanting adult heart cells have largely failed because the new cells die off quickly and do not self-renew, meaning their ability to repopulate a diseased heart is limited. Additionally, only one type of heart cell—cardiomyocytes, or beating heart muscle cells—are typically used in transplants, but a heart requires all three types of cells to heal and function properly. Injecting non-cardiac stem cells into the heart has also had limited success at treating heart failure. This is because the injected cells are not effective at transitioning into heart cells, as they require complex signals to make the change, which are absent in an adult heart. Transplanting non-cardiac stem cells also increases the risk for tumor formation, as many of the cells turn into other cell types besides the target heart cells. ieCPCs avoid this problem as they are already locked into their fate of becoming heart cells.
In the current study, 90% of ieCPCs injected and retained in a mouse heart after a heart attack successfully transformed into functioning heart cells, beating with the existing cells and creating new blood vessels. The ieCPCs significantly improved heart function, causing the heart to pump more efficiently, and the benefits lasted for at least three months. Because these cells are generated from skins cells, it opens the door for personalized medicine, using a patient’s own cells to treat their heart disease.
“Cardiac progenitor cells could be ideal for heart regeneration,” said senior author Sheng Ding, PhD, a senior investigator at Gladstone. “They are the closest precursor to functional heart cells, and, in a single step, they can rapidly and efficiently become heart cells, both in a dish and in a live heart. With our new technology, we can quickly create billions of these cells in a dish and then transplant them into damaged hearts to treat heart failure.”
Roddenberry Gift Ushers Gladstone Stem Cell Research into the Future
Roddenberry Gift Ushers Gladstone Stem Cell Research into the Future
The path to medical breakthroughs lies in bold, ambitious science
Donor Stories Institutional News Spinal Cord Injuries Alzheimer’s Disease Diabetes Roddenberry Stem Cell Center Bruneau Lab Ding Lab Huang Lab McDevitt LabResearchers Create First Stem Cells Using CRISPR Genome Activation
Researchers Create First Stem Cells Using CRISPR Genome Activation
Activating a single gene is sufficient to change skin cells into stem cells.
News Release Research (Publication) Cardiovascular Disease Ding Lab CRISPR/Gene Editing Stem Cells/iPSCsStudy Reveals How to Reprogram Cells in our Immune System
Study Reveals How to Reprogram Cells in our Immune System
The discovery could improve treatments for autoimmune diseases and cancer
Ding Lab Stem Cells/iPSCs Immunology