Gladstone NOW: The Campaign Join Us on the Journey✕

New study from Bruce Conklin (left), Melanie Ott (center), and Todd McDevitt (right) may shed light on the long-term consequences for COVID-19 patients.
Update: The researchers' findings were published in a peer-reviewed journal, Science Translational Medicine, on March 15, 2021. This piece was originally posted on August 25, 2020. This article has been updated to reflect the publication in the new journal.
COVID-19 was initially identified as a respiratory disease, but scientists now appreciate that it also affects several other organs in the body, including the heart. Heart damage is a major determinant of COVID-19 related deaths, and even patients who experience only mild COVID-19 symptoms exhibit signs of cardiac dysfunction several months after recovery.
A new study by scientists at Gladstone Institutes helps explain how SARS-CoV-2, the virus that causes COVID-19, inflicts damage on heart cells. The team’s findings, initially shared on bioRxiv and later published in Science Translational Medicine, show the virus’s unexpected effects on the structure of heart cells in the lab, as well as in heart tissue from COVID-19 patients.
The team, led by Gladstone Senior Investigators Todd C. McDevitt, PhD, and Bruce R. Conklin, MD, was uniquely positioned to tackle this work, due to their experience in deriving various types of cardiac cells in the lab from induced pluripotent stem cells.
In collaboration with Director of the Gladstone Institute of Virology Melanie Ott, MD, PhD, they exposed the cells to varying doses of SARS-CoV-2. The virus only productively infected the cardiomyocytes, or heart muscle cells, meaning that the virus could enter those cells and make new copies of itself.
“Early on in our experiments, we noticed that many of the cardiomyocytes were exhibiting some very strange features,” says McDevitt, who is also a professor of bioengineering and therapeutic sciences at UC San Francisco (UCSF). “What we were seeing was completely abnormal; in my years of looking at cardiomyocytes, I had never seen anything like it before.”
The team observed that when they exposed cardiomyocytes to SARS-CoV-2, the sarcomeres in some of the cells appeared to be diced into small, regularly sized fragments. Typically, sarcomeres—units of the muscle fibers in heart cells—are organized into long filaments aligned in the same direction. These sarcomeres control the coordinated contraction of heart cells to produce the normal heartbeat.
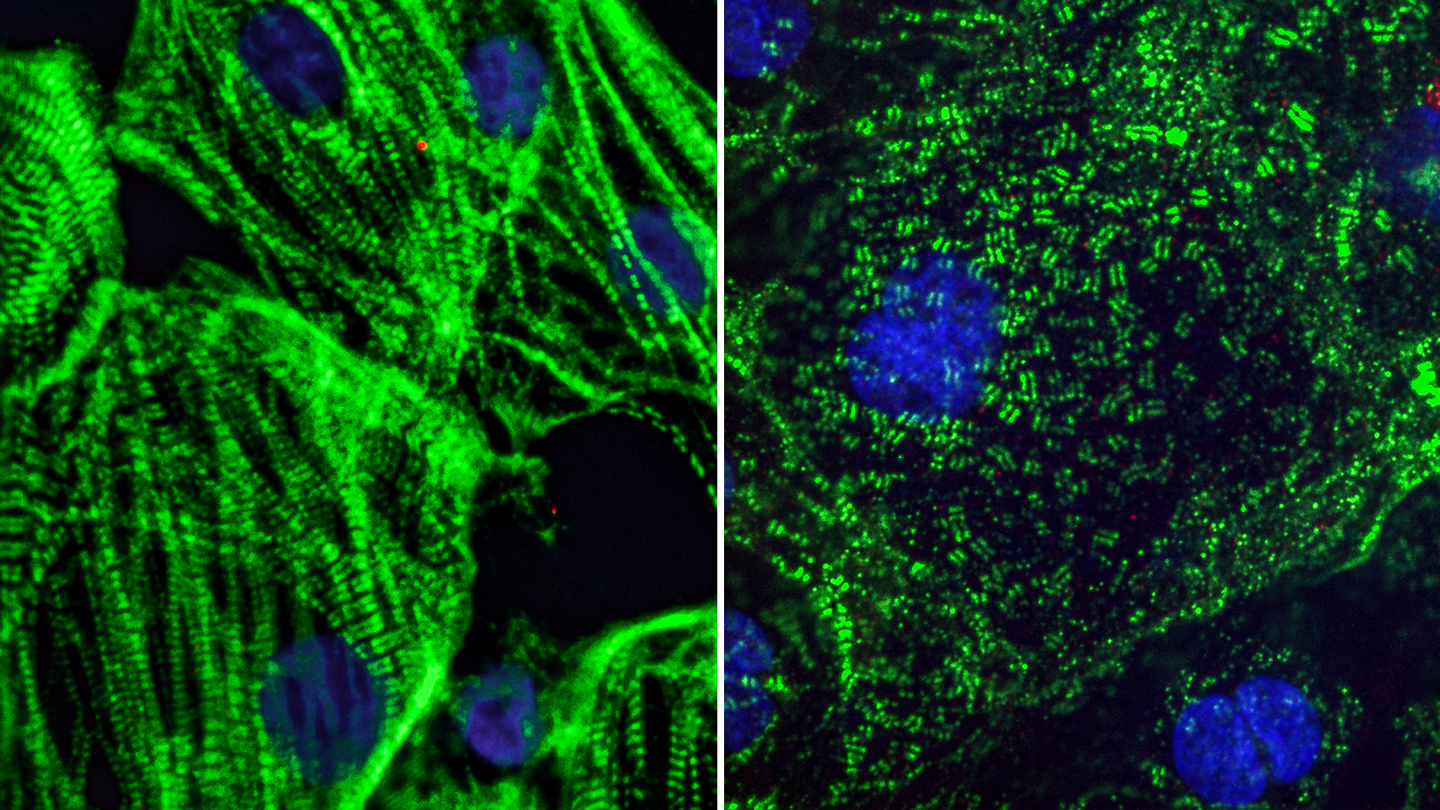
Healthy heart muscle (left) created from adult stem cells have long fibers which allow them to contract. SARS-CoV-2 infection causes these fibers to break apart into small pieces (right), which can cut off the cells ability to beat and may explain lasting cardiac defects in COVID-19 patients.
“The sarcomere disruptions we discovered would make it impossible for the heart muscle cells to beat properly,” explains Conklin, who is also a professor of medicine, cellular and molecular pharmacology, and ophthalmology at UCSF.
The scientists also noted that the nuclear DNA seemed to be missing from many of the heart cells. Without DNA, cells can no longer perform any normal functions.
“It’s the cell equivalent of being brain dead,” adds Conklin. “Even after scouring scientific literature and conferring with colleagues, we cannot find these abnormal cell features in any other cardiac disease model. We believe they are unique to SARS-CoV-2 and could explain the prolonged heart damage seen in many COVID-19 patients.”
Discoveries in a Dish Predict Changes in Human Tissue
To understand whether these changes to cells in culture were relevant to COVID-19 in humans, the researchers sought out heart tissue from COVID-19 patients. However, patient tissue was hard to find.
“Most people don’t appreciate how difficult it has been to access patient samples,” says McDevitt. “Since this virus is so contagious, many hospitals lack the special equipment they need to safely perform autopsies on COVID-19 patients.”
When the team was able to receive patient samples, what they saw corroborated the structural changes they saw in the lab. Remarkably, even in patients who had not been diagnosed with COVID-19 related heart disease, there was evidence of structural abnormalities in the heart muscle cells. Additional testing needs to be done to validate these findings further, but the immediate similarities are striking.
“These abnormalities haven’t been identified in patients before, so they may have been overlooked,” says McDevitt. “I hope our work motivates doctors to review their patients’ samples to start looking for these features at a higher magnification, which will be the true test of our hypotheses.”
Heart Cells Can Serve as a New Drug Screen
In addition to providing more insight into the impact of COVID-19 on the heart, the team’s model could be used as a novel way to test and screen drugs that could help mitigate the effects of COVID-19 in the heart, as well as other tissues susceptive to SARS-CoV-2 infection.

The team behind the new study include (left to right) Serah Kang, Juan Pérez-Bermejo, Sarah Rockwood, Camille Simoneau, Gokul Ramadoss, and David Joy.
“Heart muscle cells are highly infectible, and we have very visually distinct signs of infection,” says Conklin. “Since we can easily see the effects of infection, we can use these cells as a first screen to find drugs that could shut down the virus or prevent it from taking over the cells.”
Long-term Consequences for COVID-19 Patients
Moreover, these findings shed light on the long-term ramifications of COVID-19. Unlike some other tissues in the body, the heart does not regenerate. So, it’s possible that someone who becomes infected with COVID-19, even a mild case, could recover and then develop heart disease years later.
“It will be important to identify a protective therapy, one that safeguards the heart from the damage we’re seeing in our models,” says McDevitt. “Even if you can’t prevent the virus from infecting cells, you could put a patient on a drug to prevent these negative consequences from occurring while the disease is present.”
For Media
Julie Langelier
Associate Director, Communications
415.734.5000
Email
About the Study
The paper, “SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19,” was published in the journal Science Translational Medicine on March 15, 2021. It was initially shared publicly on bioXriv on August 25, 2020.
Other authors include Juan A. Pérez-Bermejo, Serah Kang, Sarah J. Rockwood, Ana C. Silva, Huihui Li, and Ken Nakamura, all of Gladstone Institutes; David A. Joy, Will R. Flanigan, Camille R. Simoneau, and Gokul N. Ramadoss, all graduate students at UCSF, training in Gladstone Institutes’ labs; and Jeffrey D. Whitman, Laboratory Medicine, UCSF.
The work and researchers involved were supported by grants from the National Institutes of Health (R01-HL130533, R01-HL13535801, P01-HL146366, and U01 ES032673-03), the National Science Foundation (NSF ERC 1648035), and gifts from the Roddenberry Foundation and Tom and Pauline Tusher.
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
Support Our COVID-19 Research Efforts
Gladstone scientists are moving quickly to respond to the coronavirus outbreak. Help us end this pandemic.
New Tool Reveals the Secrets of HIV-Infected Cells
New Tool Reveals the Secrets of HIV-Infected Cells
Developed by Gladstone scientists, HIV-seq could uncover new opportunities for treating HIV.
News Release Research (Publication) HIV/AIDS Infectious Disease Roan LabVitamin B3 Therapy Offers Hope for Fatal Childhood Disease
Vitamin B3 Therapy Offers Hope for Fatal Childhood Disease
A new framework that matches vitamins with genetic diseases helped uncover that high-dose vitamin B3 can dramatically extend survival in mice with NAXD deficiency.
News Release Research (Publication) Rare Diseases Jain LabGladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Gladstone Scientist Nadia Roan Elected to American Academy of Microbiology
Roan has made great strides in understanding how persistent viruses including HIV cause disease and how immunity to viruses shapes human health.
Awards News Release COVID-19 HIV/AIDS Infectious Disease Roan Lab





