Gladstone NOW: The Campaign Join Us on the Journey✕

Sheng Ding, PhD, has discovered how to turn human skin cells into insulin-producing pancreatic cells. The discovery could revolutionize diabetes research and treatment. [Photo: Chris Goodfellow, Gladstone Institutes]
Scientists at the Gladstone Institutes and the University of California, San Francisco (UCSF) have successfully converted human skin cells into fully-functional pancreatic cells. The new cells produced insulin in response to changes in glucose levels, and, when transplanted into mice, the cells protected the animals from developing diabetes in a mouse model of the disease.
The new study, published in Nature Communications, also presents significant advancements in cellular reprogramming technology, which will allow scientists to efficiently scale up pancreatic cell production and manufacture trillions of the target cells in a step-wise, controlled manner. This accomplishment opens the door for disease modeling and drug screening and brings personalized cell therapy a step closer for patients with diabetes.
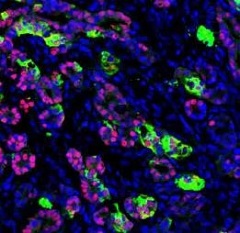 Functioning human pancreatic cells after they’ve been transplanted into a mouse. [Image: Saiyong Zhu, Gladstone Institutes]
Functioning human pancreatic cells after they’ve been transplanted into a mouse. [Image: Saiyong Zhu, Gladstone Institutes]
“Our results demonstrate for the first time that human adult skin cells can be used to efficiently and rapidly generate functional pancreatic cells that behave similar to human beta cells,” says Matthias Hebrok, PhD, director of the Diabetes Center at UCSF and a co-senior author on the study. “This finding opens up the opportunity for the analysis of patient-specific pancreatic beta cell properties and the optimization of cell therapy approaches.”
In the study, the scientists first used pharmaceutical and genetic molecules to reprogram skin cells into endoderm progenitor cells—early developmental cells that have already been designated to mature into one of a number of different types of organs. With this method, the cells don’t have to be taken all the way back to a pluripotent stem cell state, meaning the scientists can turn them into pancreatic cells faster. The researchers have used a similar procedure previously to create heart, brain, and liver cells.
After another four molecules were added, the endoderm cells divided rapidly, allowing more than a trillion-fold expansion. Critically, the cells did not display any evidence of tumor formation, and they maintained their identity as early organ-specific cells.
The scientists then progressed these endoderm cells two more steps, first into pancreatic precursor cells, and then into fully-functional pancreatic beta cells. Most importantly, these cells protected mice from developing diabetes in a model of disease, having the critical ability to produce insulin in response to changes in glucose levels.
“This study represents the first successful creation of human insulin-producing pancreatic beta cells using a direct cellular reprogramming method,” says first author Saiyong Zhu, PhD, a postdoctoral researcher at the Gladstone Institute of Cardiovascular Disease. “The final step was the most unique—and the most difficult—as molecules had not previously been identified that could take reprogrammed cells the final step to functional pancreatic cells in a dish.”
Sheng Ding, PhD, a senior investigator in the Roddenberry Stem Cell Center at Gladstone and co-senior author on the study, adds, “This new cellular reprogramming and expansion paradigm is more sustainable and scalable than previous methods. Using this approach, cell production can be massively increased while maintaining quality control at multiple steps. This development ensures much greater regulation in the manufacturing process of new cells. Now we can generate virtually unlimited numbers of patient-matched insulin-producing pancreatic cells.”
Roddenberry Gift Ushers Gladstone Stem Cell Research into the Future
Roddenberry Gift Ushers Gladstone Stem Cell Research into the Future
The path to medical breakthroughs lies in bold, ambitious science
Donor Stories Institutional News Spinal Cord Injuries Alzheimer’s Disease Diabetes Roddenberry Stem Cell Center Bruneau Lab Ding Lab Huang Lab McDevitt LabScientists Discover Drug that Increases “Good” Fat Mass and Function
Scientists Discover Drug that Increases “Good” Fat Mass and Function
Anti-cancer drug prevented less weight gain and caused mice to burn more calories thanks to higher levels of metabolism-boosting brown fat.
Ding Lab Metabolism DiabetesBrain Receptor Regulates Fat Burning in Cells
Brain Receptor Regulates Fat Burning in Cells
Scientists at the Gladstone Institutes have discovered an unusual regulator of body weight and the metabolic syndrome: a molecular mechanism more commonly associated with brain cells.
Diabetes Neurological Disease Akassoglou Lab Basic Science Metabolism