In the latest issue of the Journal of Neuroscience, Drs. Steve Finkbeiner and Gaia Skibinski harness the power of their robotic microscope to demonstrate how a unique protein interaction may drive the most common genetic cause of Parkinson’s disease. [Photo: Chris Goodfellow]
You’re trying to piece together the life story of an individual and all you have to go on is a few photographs. But that’s not even the hard part. Because each photograph also contains several thousand other people, the person you’re looking for is just one among a sea of faces. What could you realistically glean about that person’s life? Could you discover where they grew up? What they were like? When they died? Could you even locate that person in the first place?
This difficult task is often the stark reality faced by scientists as they work tirelessly to understand what happens to individual cells that become ravaged by disease. And it is especially true when studying diseases of the brain, such as Alzheimer’s, Parkinson’s and Huntington’s—neurodegenerative diseases that are defined by their capability to attack individual neurons and thus disrupt healthy brain function. In a sort of molecular “Where’s Waldo,” scientists are often left with no choice but to measure a disease’s effects on populations of cells, rather than a single cell. The end result is often just a partial understanding of the disease—and represents a major barrier toward developing effective treatments.
This was the problem that faced Gladstone Neuroscientist Steve Finkbeiner, MD, PhD, several years ago. But armed with ingenuity and determination, he invented a one-of-a-kind robotic microscope with the unique ability to track individual neurons as they grow, develop, mature and eventually succumb to disease. Now in its third incarnation, the microscope has helped Dr. Finkbeiner and his team advance research in many fields of neuroscience. In the team’s latest efforts, published today in the Journal of Neuroscience, they harness the power of the microscope to uncover new insights into Parkinson’s disease.
Revealing the Invisible
The most devastating aspect of Parkinson’s disease may not be its debilitating symptoms, which rob its victims of their ability to control their own movement. It may not be the millions around the world and their families who suffer each day from the disease’s harmful effects. Instead, it may in fact be that its root causes remain largely a mystery. But in their latest research, Dr. Finkbeiner and his research team, including Gladstone Staff Research Scientist Gaia Skibinski, have focused their attention on LRRK2—the most common genetic cause of Parkinson’s. They find that LRRK2’s unique relationship with another brain protein may actually fuel Parkinson’s progression. And in so doing, they also shed light on a potential strategy to disrupt this toxic process.
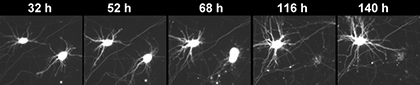
The consecutive images are of two neurons, imaged each day for five days. The top neuron does well throughout the five days. However the lower neuron begins to degenerate on the third day. And by the fourth day, it is gone. By longitudinally tracking neurons the Gladstone research team has much greater power at picking up differences in neurons that had mutant LRRK2, and those that did not. [images: Gaia Skibinski]
Scientists have long known that mutations in LRRK2 cause misfolded versions of the LRRK2 protein to accumulate in neurons. The prevailing hypothesis has been that misfolded LRRK2 boosts the activity of a type of enzyme called kinase, and that this heightened kinase activity is what drives cell death. Scientists have also looked to the fact that mutant LRRK2 tends to clump together into so-called inclusion bodies (IBs) as another contributor to the disease’s progression.
“As a result, researchers have used the presence of IBs and heightened kinase activity as a proxy for measuring LRRK2’s harmful effects, rather than measuring LRRK2 levels directly,” explained Dr. Finkbeiner. “But we were unconvinced that these were the main drivers of cell death—so we decided to take a closer look at what was happening inside the cell.”
Challenging Conventional Wisdom
The researchers generated neurons from two sources. First, from rats genetically modified to have mutant LRRK2. The second source, human neurons derived from the skin cells of LRRK2-related Parkinson’s patients, utilized the latest induced pluripotent stem cell (iPSC cell) technology. This technique, pioneered by Gladstone Investigator and 2012 Nobel Laureate Shinya Yamanaka MD, PhD, allows scientists to reprogram adult skin cells into cells that are virtually identical to stem cells. These stem cells can then develop into almost any cell in the body.
For this study, the newly generated, patient-derived neurons exhibited the same pattern of cell degeneration and death that occurs inside a patient’s brain. Studying these models allowed the research team to see how the LRRK2 mutation kick starts neurodegeneration—making this an extremely powerful model of Parkinson’s disease.
Using the robotic microscope, they then tracked the human and rat cells over time, monitoring the buildup of mutant LRRK2 and how that buildup was tied to cell death.
“In both models, we found that neither IBs nor kinase activity were the direct causes of cellular toxicity leading to neuronal death,” said Dr. Skibinski, who was the paper’s lead author. “Instead, the underlying cause appeared to be tied directly to the accumulation of diffuse, mutant LRRK2.”
But as the research team peered even closer into the lives of these neurons, they found something intriguing: a unique interplay between mutant LRRK2 and alpha-synuclein, another protein that has long been associated with Parkinson’s. Past research has shown that Parkinson’s patients with LRRK2 mutations often show an abnormal accumulation of alpha-synuclein as well. But until now, the exact nature of the relationship between these two proteins remained unclear.
“Importantly, we found that in both the rat and human cellular models, removing alpha-synuclein reduced LRRK2-related cell death—which was completely unexpected,” continued Dr. Skibinski. “When we looked closer, we found that the removal of alpha-synuclein led to an immediate drop in levels of LRRK2. To our knowledge, this is the first demonstration that this type of neurodegeneration can be halted, by simply eliminating alpha-synuclein.”
The team hypothesizes that, in patients with mutant LRRK2, the build-up of alpha-synuclein hinders the cell’s ability to clear away the LRRK2, and so it accumulates. Over time, the buildup of mutant LRRK2 becomes toxic to the cell, and the cell dies.
“Our discovery of this ‘synergy’ between two proteins long known to play a role in Parkinson’s is a huge step towards developing drugs that attack the disease’s underlying mechanisms,” said Dr. Finkbeiner. “As we continue to unravel the precise functional relationship between alpha-synuclein and LRRK2, we are well on our way to halting the onslaught of Parkinson’s on the brain.”
Meet Gladstone: Christina Buselli
Meet Gladstone: Christina Buselli
Research Associate Christina Buselli describes her work in the Finkbeiner lab, her path to science, and the questions she wishes she could ask her grandfather
Profile Finkbeiner LabWhen Two Disruptive Technologies Converge
When Two Disruptive Technologies Converge
How gene editing and stem cells are working in tandem at Gladstone Institutes to change science and medicine
Deep Dive Stem Cells/iPSCs CRISPR/Gene Editing Srivastava Lab Yamanaka Lab Doudna Lab Conklin Lab Marson Lab Huang Lab Ott LabWorld’s Largest Public ALS Data Portal Launched
World’s Largest Public ALS Data Portal Launched
Steve Finkbeiner is leading key components of this unprecedented resource to boost ALS research.
Gladstone Experts ALS Center for Systems and Therapeutics Finkbeiner Lab AI Robotic Microscopy Stem Cells/iPSCs